REVIEW
Rational
pharmacological approach to clinical studies for the treatment of SARS-COV-2
infection
Antônia Dailane dos Santos Rabêlo*, Gizelle Gomes de Souza*, Rosilene Ribeiro de Sousa**, Charllyton Luis Sena da Costa, D.Sc.***
*Undergraduate
from Nursing Department, Centro Universitário Santo Agostinho,
Teresina/PI, Brazil, **Especialista em Farmácia
Clínica, Instituto Brasil de Pós-Graduação, Teresina/PI, Brazil,
***Doutor em Biotecnologia pela RENORBIO, Pharmacy Department, Centro Universitário Santo Agostinho,
Teresina/PI, Brazil
Received
on: August 18; 2020; Accepted on: August 21; 2020.
Corresponding author: Charllyton
Luis Sena da Costa, Rua Miguel Arcoverde, 191/203,
Bloco C, Bairro Noivos, 64048-330 Teresina PI
Antônia Dailane dos Santos Rabêlo: daylanerabelo199519@gmail.com
Gizelle Gomes de Souza:
gizellegomes1901@gmail.com
Rosilene Ribeiro de
Sousa: rosersena@gmail.com
Charllyton Luis
Sena da Costa : charllyton@gmail.com
Abstract
The
global emergency generated by the COVID-19 pandemic caused by the SARS-COV-2
virus has created serious impacts on the different populations of the planet
and has triggered the generation of scientific information on an unprecedented
scale until then for a single topic. One of the consequences of the global
scientific effort lies in the large number of substances already tested, by
different methods, the search for an effective treatment for the infection and
the consequent disease, remaining without absolute success so far. Assimilating
the lessons, learned from the successful adoption of therapies combining
multiple drugs used in HIV infection, the evidence obtained from the large
amount of published information regarding the action of many substances with
different mechanisms, now allows the proposition, in this work, of tests
clinical trials for the evaluation of regimens composed of at least three drugs
in combination. Rational combination schemes can target different molecular
components of the virus affecting different points in the SARS-COV-2
replication cycle, such as virus fusion to the host cell, replication and viral
particle assembly generating a potentially more effective synergistic effect
than attempts using a single substance.
Keywords: antiviral, pandemic, combination therapy.
Resumo
Abordagem farmacológica
racional para estudos clínicos para o tratamento da infecção por SARS-COV-2
A emergência mundial
definida pela pandemia de COVID-19 causada pelo vírus SARS-COV-2 gerou graves
impactos nas diferentes populações do planeta e desencadeou a geração de informações
científicas sobre um único tema numa escala sem precedentes até então. Uma das
consequências do esforço científico global reside no grande número de
substâncias que já foram avaliadas, por diferentes métodos, a busca de um
tratamento eficaz para a infecção da doença consequente, permanecendo sem
sucesso absoluto até o momento. Assimilando os aprendizados da adoção,
bem-sucedida, de terapêuticas combinando múltiplos fármacos utilizados na
infecção pelo HIV, as evidências obtidas da grande quantidade de informação
publicada relativas a ação de muitas substâncias com diferentes mecanismos,
permite agora a proposição, neste trabalho, de testes clínicos para a avaliação
de esquemas compostos por no mínimo três fármacos em combinação. Os esquemas
racionais de combinação podem alvejar diferentes componentes moleculares do
vírus afetando diferentes pontos do ciclo de replicação do SARS-COV-2, como a
fusão do vírus à célula hospedeira, replicação e montagem da partícula viral
gerando efeito sinérgico potencialmente mais eficaz do que as tentativas
utilizando uma única substância por vez vista até então.
Palavras-chave: antiviral, pandemia,
terapia de combinação.
Resumen
Enfoque farmacológico
racional de los estudios
clínicos para el tratamiento
de la infección por
SARS-COV-2
La emergencia
global generada por la
pandemia COVID-19 provocada por el virus SARS-COV-2 ha creado graves
impactos en las diferentes poblaciones del planeta y ha desencadenado la generación de información científica a una escala sin
precedentes hasta entonces para un
solo tema. Una de las consecuencias
del esfuerzo científico
global radica en la gran cantidad de sustancias ya testadas, por
diferentes métodos, la búsqueda
de un tratamiento eficaz
para la infección y la consecuente enfermedad, quedando sin éxito hasta el momento. La asimilación de las lecciones
aprendidas de la exitosa adopción
de terapias que combinan múltiples
fármacos utilizados en la infección por VIH, la evidencia obtenida de la gran cantidad
de información publicada sobre la
acción de muchas sustancias con diferentes
mecanismos, permite ahora proponer,
en este trabajo, pruebas. Ensayos clínicos para la evaluación de regímenes compuestos por al menos tres
fármacos en combinación.
Los esquemas de combinación racional pueden apuntar a diferentes
componentes moleculares del virus
que afectan diferentes puntos
en el ciclo de replicación del SARS-COV-2, como la fusión del
virus a la célula huésped, la replicación
y el ensamblaje de
partículas virales, generando un
efecto sinérgico potencialmente más efectivo que los intentos de usar
un solo sustancia.
Palabras-clave: antiviral, pandemia,
terapia de combinación.
Introduction
The emergence and rise of the new coronavirus (SARS-COV-2), culminating
in its spread on a pandemic scale, and generating unthinkable losses in human
lives lost or severely affected by the disease resulting from the infection
(COVID-19). This clearly generated a movement global in the scientific
community towards understanding the new pathology as well as materialized in
the search for effective forms of treatment [1]. This movement strengthened the
efforts to fight the disease and generated a very large amount of information
in a short period between the end of 2019 and July 2020. The number of
citations to studies referenced in Pubmed until the
end of July 2020 is 17,782 and by the end of 2019, there were only 10.
The enormous volume of published studies clearly reflects the great
effort and dedication of researchers around the world to produce knowledge that
is capable of contributing to reducing the impacts of
the pandemic on the different populations of the globe. One of the main aspects
guiding the efforts dedicated to COVID-19 is directed to studies related to the
development of new molecules endowed with absolute efficacy, until then not
achieved, and the evaluation of molecular species already belonging to the
therapeutic arsenal of humanity in search of the already mentioned efficacy
[2-4]. The use of existing drugs in the context of treatment strategies adopted
for aggravated cases followed a priori the paths of the clinical experience of
professionals in the care of patients with COVID-19 and under the premise of
using off label of pharmacological resources already available. Such an
approach defined the demands for conducting clinical studies necessary to build
a solid basis for the ostensibly safe use of treatment protocols and that have
efficacy to compensate for the risks inevitably inherent in any pharmacological
treatment [5,6].
Many molecules have already been tested in different assessment
situations and numerous are the promising individual cases that give rise to
hopes for solving the central problem. Less common, however, are initiatives
for rational assessment of treatment schemes based on the use of multiple drug
therapy acting on different molecular systems of the target microorganism
[7,8]. In this review, we propose the possibility of evaluating the efficacy
and safety of pharmacological schemes combining different therapeutic agents in
a synergistic way for the treatment of SARS-COV-2 infection in analogy to the
combination antiretroviral therapy protocol used in HIV infection [9,10].
Methods
This work was performed as guided to the Preferred Reporting Items for
Systematic Reviews and Meta-Analysis (PRISMA) statement. PubMed database was
searched for studies concern pharmacological propositions related to COVID-19
through July 2020 without restrictions of time and language but limited to
Clinical Trial filter. SARS-COV-2 and COVID-19 were the searching terms used.
The studies included in this work had to fulfill the following criteria:
1) Submit a clinical evaluation of one or more substances for the treatment of
SARS-COV-2 infection. 2) Works not focused on the evaluation of palliative
treatments, support or with natural products. One researcher extracted data and
other one reviewed it from selected papers, to minimize discrepancies.
Results
and discussion
The
PRISMA flow diagram (Figure 1) presents records of reviewing process that
result on 15 works after 54 originally returned on initial search.
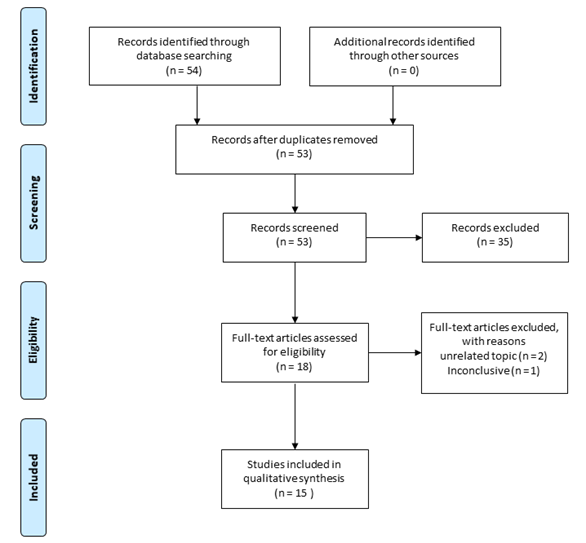
Figure
1 - PRISMA flow diagram for systematic selection of
works.
Therapeutic
approach to HIV infection
Many lessons offered, over the years, by the effort to develop specific
drugs capable of contributing to the reduction of HIV viral load in patients.
Among the legacies of this process, it is necessary to highlight the impact
generated by the introduction of HIV protease inhibitors on the success of
treatment [11-13], but also by highlighting the evident superiority of
therapeutic schemes based on multiple drugs compared to strategies centered on
use of a single drug or pharmacological category. The drug approach aimed at
multiple molecular targets in HIV [14-16], thus allows a synergistic effect and
reduces the scope of adaptive adjustments of viral metabolism to attacks
produced by a single drug on a single target.
The drugs allocated in Table I, which are part of the main therapeutic
regimens used in HIV combined antiretroviral therapy in current clinical
practice. The therapeutic regimes presented here are organized as present in
the WHO Guidelines [17] updated in December 2018 and adapted for this work. The
different drug regimens are in order of preference for the initiation of
treatment, as well as the relation of indication of each regimen by population
groups considering gender, age group and reproductive capacity.
Table
I - Summary of the reference therapeutic regimes,
proposed by the WHO, applicable to the treatment of patients with HIV infection.
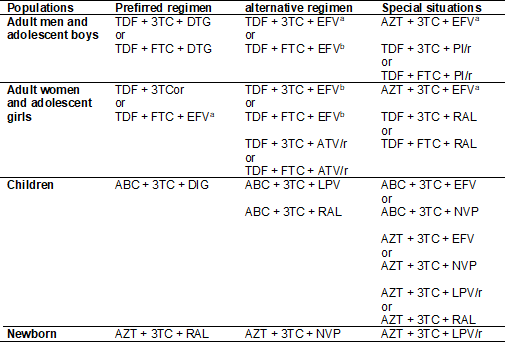
Abacavir
(ABC), Emtricitabine (FTC), Lamivudine (3TC), Zidovudine (AZT), enofovir disoproxil fumarate (TDF), Efavirenz (EFV),
Nevirapine (NVP), Atazanavir/ritonavir (ATV/r), Lopinavir/ritonavir (LPV/r),
Dolutegravir (DTG), Raltegravir (RAL). a = 600 mg; b
= 400 mg
In a quick analysis of the information summarized in Table I on the
therapeutic classes that contribute to the composition of the different
anti-HIV therapeutic regimens, it is possible to observe representatives of the
following classes: Nucleoside Inhibitors of Reverse Transcriptase, Nucleotide
Inhibitors of Reverse Transcriptase, Non-Nucleoside Inhibitors of Reverse
transcriptase, Protease Inhibitors and Integrase Inhibitors. In this way, there
are five different classes of molecules with pharmacological activities
centered on the action on three different molecular targets critical to the HIV
infection cycle.
The undisputed success of antiretroviral therapy in reducing viral load,
to almost undetectable levels, in HIV-infected patients is, of course, also a
consequence of the combined use of different molecules acting in synergy to
reduce viral replication [18,19]. Evidence of the value of the combination
therapy can be seen in the composition of the 28 therapeutic regimens present
in Table I. The enzyme Reverse Transcriptase is clearly the preferred molecular
target for all the therapeutic regimens listed, since Reverse Transcriptase
Inhibitors contribute two of the three drugs in all triple regimens and are
half of those with four drugs. Still, none of the therapeutic approaches listed
in Table I fails to use drugs targeting other molecular components of the virus
in the successful intention of extending their antiviral efficacy.
The use of drugs in the class of HIV Protease Inhibitors and Integrase
inhibitors, as well as the use of more than 1 Reverse Transcriptase Inhibitor
per therapeutic regimen reinforce the argument. A therapeutic strategy centered
on action on multiple targets using different drugs is a logical path for
proposing clinical studies aimed at evaluating treatment strategies for viral infections
causing illnesses that had not been treated [20].
Candidates
for clinical studies to define a combined pharmacological approach for
SARS-COV-2.
Despite the evidence of success presented by the strategy of combining
drugs with multiple mechanisms of action for the treatment of HIV infection,
only 1 of the 54 works classified in the PubMed as clinical trial (1,85%),
until July 2020 ends, presented more than two drugs as a treatment alternative
for SARS-COV-2 infection, one of which is interferon beta-1b [21-25]. While
chloroquine or hydroxychloroquine were evaluated in 7 of 54 studies in the same
category (12.96%).
The only study using a triple drug regimen, containing interferon
beta-1b, Lopinavir-ritonavir and ribavirin, found statistically significant
results in reducing symptoms and positive findings from tests with
nasopharyngeal swabs. Another point to highlight is the absence of significant
findings when considering the adverse events of the group undergoing treatment
with the triple regimen in comparison with the control group, allowing to
ponder that the increase in therapeutic agents did not result in automatic
elevation of risks in the study. This result, although solitary among clinical
studies, testifies in favor of the argument of the superiority of multidrug
regimens, and the need for more clinical tests for drug combination regimens,
for the treatment of viral infections, since in that study the group the
control used only the protease inhibitor Lopinavir-ritonavir [21], which is
part of treatment protocols against HIV. This characteristic is further
reinforced by the inability to produce statistically significant results in clinical
studies that independently used the drugs Lopinavir-ritonavir [22], Remdesivir [22,23], Ruxolitinib
[24] and Oseltamivir [25], although the findings in all these studies indicated
improvements in the clinical conditions of patients undergoing different
treatments with each drug.
Because of the focus of the global scientific community on attempts to
understand and resolve the disease caused by SARS-COV-2, many drugs have been
tested against the microorganism and different mechanisms of action and
molecular targets have been determined [26-28]. In Table II, some drugs are
grouped by strategy of action on the virus and can be used in the definition of
multiple schemes for clinical testing aiming at the evaluation of efficacy and
safety and then their possible uses in treatment strategies aiming at the
reduction of viral load and consequently of typical COVID-19 characters.
Table
II - Candidate drugs for conducting clinical studies
to build multiple antiviral regimens for treating SARS-VOC-2 infection grouped
by strategy of action on viral infection cycle.

Table II brings together drugs that have different specific molecular
targets and can affect three critical steps for the viral replication: cycle,
Cell entry, replication of the virus genetic material and assembly of the viral
particles. The activity assignments of the molecules listed in the table are
widely documented [2,8,21,29-32,34], and represent a strategy predominantly
based on targeting viruses, in which the molecular targets of the drugs listed
belong primarily to the virus and not to the host, as well as the commercial
availability of the drugs presented.
Considering the drugs listed in Table II that act by interfering with
the entry of the virus into cells, there are three different molecular
mechanisms to be explored in proposals for combined antiviral treatment schemes
for SARS-COV-2. In the column of drugs that act to affect the replication
capacity of the virus RNA, there are drugs that can affect the action of
RNA-dependent RNA polymerase (RdRp) and that can
compose a possible therapeutic scheme combined one at a time or with more than
one representative. The column, in table 2, containing drugs capable of
interfering with the assembly of the viral particles. In this category are the
representatives with the fewest direct evidence of application on SARS-COV-2 so
far, however, preliminary studies have pointed out the possibility that the
listed molecules inhibit the main protease of the virus, which is responsible
for post-synthesis processing of polypeptides translated from viral RNA
[2,8,29].
A rational, and potentially effective, pharmacological treatment
strategy focused on reducing the number of viral particles in infected
individuals could use at least one representative from each group shown in
Table II. The proposal for clinical tests to evaluate efficacy and safety of
various combinations of drugs capable of acting in different biochemical
systems, linked to the viral life cycle, can generate a synergistic effect in
order to increase their chances of success in the treatment of infected
patients. The definitions of combined therapeutic schemes to be tested should
not be restricted to the examples of drugs presented in Table II but should consider
the presence of molecules capable of simultaneously affecting the functioning
of multiple molecular mechanisms critical for viral replication previously
known.
Conclusion
The intense work of many researchers around the world has generated
strong evidence related to the action of many drugs on different molecular
targets associated with the SARS-COV-2 infection cycle. Thus, we propose the
clinical evaluation of combined antiviral regimens containing at least three
drugs as a rational alternative to be considered, as opposed to single-drug
regimens for the treatment of SARS-COV-2 infection, which had not yet been
effective.
References
- World
Health Organization. COVID-19 Global Research Roadmap: 2019 Novel coronavirus.
Geneva, Switzerland: WHO; 2020.
- Li
H, Zhou Y, Zhang M, Wang H, Zhao Q, Liu J. Updated approaches against
SARS-CoV-2. Antimicrob Agents Chemother 2020;64(6):e00483-20.
https://doi.org/doi.org/10.1128/AAC.00483-20
- Hussain
S, Xie YJ, Li D, Malik SI, Hou JC, Leung EL et al. Current
strategies against COVID-19. Chinese Medicine 2020;15(70).
https://doi.org/10.1186/s13020-020-00353-7
- Li
G, De Clercq E. Therapeutic options for the 2019
novel coronavirus (2019-nCoV). Nat Rev Drug Discov
2020;19(3):149-50. https://doi.org/10.1038/d41573-020-00016-0
- Cao
B, Wang Y, Wen D, Liu W, Wang J, Fan G et al. A trial of lopinavir-ritonavir in
adults hospitalized with severe covid-19. New Engl J
Med 2020;382(19):1787-99. https://doi.org/10.1056/NEJMoa2001282
- Molina
JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L et al. No
evidence of rapid antiviral clearance or clinical benefit with the combination
of hydroxychloroquine and azithromycin in patients with severe COVID-19
infection. Med Mal Infect 2020;50(4):384. https://doi.org/10.1016/j.medmal.2020.03.006
- Caly L, Druce
JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved
drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020;178:104787.
https://doi.org/10.1016/j.antiviral.2020.104787
- Lotfi M, Hamblin MR, Rezaei N. COVID-19:
Transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta 2020;508:254-66.
https://doi.org/10.1016/j.cca.2020.05.044
- Maenza J, Flexner C. Combination
antiretroviral therapy for HIV infection [published correction appears in Am
Fam Physician 1998;58(5):1084. Am Fam Physician 1998;57(11):2789-98.
- Eggleton JS, Nagalli S. Highly Active Antiretroviral Therapy (HAART)
[Updated 2020 Jul 5]. Treasure Island FL: StatPearls;
2020.
- Wensing AM, van Maarseveen NM, Nijhuis M. Fifteen years of HIV Protease
Inhibitors: raising the barrier to resistance. Antiviral Res 2010;85(1):59-74.
https://doi.org/10.1016/j.antiviral.2009.10.003
- Broder
S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS
pandemic. Antiviral Res 2010;85(1):1-18.
https://doi.org/10.1016/j.antiviral.2009.10.002
- Lv Z, Chu Y, Wang Y. HIV protease
inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl) 2015;7:95-104.
https://doi.org/10.2147/HIV.S79956
- Powderly
WG. Integrase inhibitors in the treatment of HIV-1 infection. J Antimicrob Chemother 2010;65(12):2485-8.
https://doi.org/10.1093/jac/dkq350
- Penazzato M, Giaquinto
C. Role of non-nucleoside reverse transcriptase inhibitors in treating
HIV-infected children. Drugs 2011;71(16):2131-49.
https://doi.org/10.2165/11597680-000000000-00000
- Moran
CA, Weitzmann MN, Ofotokun
I. The protease inhibitors and HIV-associated bone loss. Curr
Opin HIV AIDS 2016;11(3):333-42.
https://doi.org/10.1097/COH.0000000000000260
- World
Health Organization. Updated recommendations on first-line
and second-line antiretroviral regimens and post-exposure prophylaxis and
recommendations on early infant diagnosis of HIV: supplement to the 2016
consolidated guidelines on the use of antiretroviral drugs for treating and
preventing HIV infection - December 2018. Geneva: WHO; 2018.
- Maeda
K, Das D, Kobayakawa T, Tamamura
H, Takeuchi H. Discovery and Development of anti-HIV therapeutic agents:
progress towards improved HIV medication. Curr Top
Med Chem 2019;19(18):1621-49. https://doi.org/10.2174/1568026619666190712204603
- Chaudhuri
S, Symons JA, Deval J. Innovation and trends in the development and approval of
antiviral medicines: 1987-2017 and beyond. Antiviral Res 2018;155:76-88.
https://doi.org/10.1016/j.antiviral.2018.05.005
- Himmel
DM, Arnold E. Non-nucleoside reverse transcriptase inhibitors join forces with
integrase inhibitors to combat HIV. Pharmaceuticals (Basel) 2020;13(6):122.
https://doi.org/10.3390/ph13060122
- Hung
IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY et al. Triple combination of
interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of
patients admitted to hospital with COVID-19: an open-label, randomised,
phase 2 trial. Lancet 2020;395(10238):1695-1704.
https://doi.org/10.1016/S0140-6736(20)31042-4
- Wang
Y, Zhang D, Du G, Du R, Zhao J, Jin Y et al. Remdesivir in adults with severe
COVID-19: a randomised, double-blind,
placebo-controlled, multicentre trial. Lancet
2020;395(10236):1569-78. https://doi.org/10.1016/S0140-6736(20)31022-9
- Antinori S, Cossu
MV, Ridolfo AL, Rech R, Bonazzetti C, Pagani G et al. Compassionate remdesivir treatment of severe Covid-19 pneumonia in
intensive care unit (ICU) and Non-ICU patients: Clinical outcome and
differences in post-treatment hospitalisation status.
Pharmacol Res 2020;158:104899.
https://doi.org/10.1016/j.phrs.2020.104899
- Cao
Y, Wei J, Zou L, Jiang T, Wang G, Chen L et al. Ruxolitinib in treatment of severe
coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized
controlled trial. J Allergy Clin Immunol 2020;146(1):137-46.e3.
https://doi.org/10.1016/j.jaci.2020.05.019
- Coenen S, van der Velden AW, Cianci D, Goossens H, Bongard E, Saville BR et al. (2020). Oseltamivir for
coronavirus illness: post-hoc exploratory analysis of an open-label, pragmatic,
randomised controlled trial in European primary care
from 2016 to 2018. BR
J Gen Pract
2020;70(696):e444–e9. https://doi.org/10.3399/bjgp20X711941
- Lu
CC, Chen MY, Lee WS, Chang YL. Potential therapeutic agents against COVID-19:
What we know so far. J Chin Med Assoc 2020;83(6):534-6.
https://doi.org/10.1097/JCMA.0000000000000318
- Park
SJ, Yu KM, Kim YI, Kim SM, Kim EH, Kim SG et al. Antiviral efficacies of
FDA-approved drugs against SARS-CoV-2 Infection in Ferrets. mBio, 2020;11(3):e01114-20. https://doi.org/10.1128/mBio.01114-20
- Yamaya M, Nishimura H, Deng X, Kikuchi A,
Nagatomi R. Protease inhibitors: candidate drugs to
inhibit severe acute respiratory syndrome coronavirus 2 replication. Tohoku J
Exp Med 2020;251(1):27-30. https://doi.org/10.1620/tjem.251.27
- McKee
DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and
COVID-19. Pharmacol Res 2020;157:104859.
https://doi.org/10.1016/j.phrs.2020.104859
- Richardson
P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A
et al. Baricitinib as potential treatment for
2019-nCoV acute respiratory disease. Lancet 2020;395(10223):e30–e31.
https://doi.org/10.1016/S0140-6736(20)30304-4
- Zhou
H, Fang Y, Xu T, Ni WJ, Shen AZ, Meng XM. Potential
therapeutic targets and promising drugs for combating SARS-CoV-2. Br J Pharmacol 2020;177(14):3147-61. https://doi.org/10.1111/bph.15092
- Boriskin
YS, Pécheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV
infection. Virol J 2006;3:56.
https://doi.org/10.1186/1743-422X-3-56
- Huang
D, Yu H, Wang T, Yang H, Yao R, Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): A
systematic review and meta-analysis ahead of print 2020 Jul 3. J Med Virol 2020;10. https://doi.org/10.1002/jmv.26256
- Beck
BR, Shin B, Choi Y, Park S, Kang K. Predicting commercially available antiviral
drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target
interaction deep learning model. Comput Struct
Biotechnol J 2020;18:784-90.
https://doi.org/10.1016/j.csbj.2020.03.025