ARTIGO ORIGINAL
Constraint-induced movement therapy in the
rehabilitation of chronic hemiparetic patients in the
Amazonia
Terapia de restrição e indução do movimento
na reabilitação de pacientes hemiparéticos crônicos na Amazônia
Larissa Salgado de
Oliveira Rocha, Ft.*, Lizandra Dias Magno, Ft.**, Luciane Lobato Sobral, Ft.*,
Rodrigo Santiago Barbosa Rocha, Ft.*, Rosana Macher Teodori, Ft., D.Sc.***
*Doutorando
em Ciências do Movimento pela Universidade Metodista de Piracicaba, **Mestranda
em Saúde na Amazônia pela Universidade Federal do Pará, Residente do programa
de Residência Multiprofissional em Oncologia da Universidade Federal do Pará,
***Fisioterapeuta e bióloga, Programa de Pós-graduação em Ciências do Movimento
Humano pela Universidade Metodista de Piracicaba
Recebido em 3 de novembro de 2016; aceito em 26 de maio de 2017.
Corresponding
author:
Larissa Salgado de Oliveira Rocha, Rua Tiradentes 740, 66060-902 Belém PA,
E-mail: lari1980@gmail.com; Luciane Lobato Sobral: lucianelobatosobral@gmail.com;
Rodrigo Santiago Barbosa Rocha: fisiorocha2000@yahoo.com.br; Lizandra Dias
Magno: lizandradmagno@gmail.com; Rosana Macher Teodori: rteodori@unimep.br
Abstract
Introduction: The stroke is one of the incident diseases in the world, causing
numerous changes to the functionality especially those related to upper limb
functions. Objective: To evaluate the
influence of modified Constraint-Induced Movement Therapy (mCIMT)
on functional recovery, range of motion (ROM) and muscle tone of chronic hemiparetic upper limb (UL). Methods: Seven subjects (52.75 ± 6.63 years old) were evaluated
before, straightaway and one month after 12 sessions of mCIMT,
by goniometry, modified Fugl-Meyer Assessment (mFMA) and modified Ashworth Scale (MAS). Results: Functionality improved 74.7%
after treatment and 79.5% one month after the end of treatment. There was
improvement in passive motion (p = 0.01), in pain (p = 0.004) and UL motor
function (p ≤ 0.001), increased range of flexion, extension, abduction and
adduction of the shoulder and flexion and radial deviation of the wrist (p =
0.05) and muscle tone reduction (p < 0.05). Conclusion: mCIMT was effective for
recovery of ROM in shoulder and wrist; recovery of the paretic UL functionality
and spasticity reduction, and the results remained after the end of treatment.
Key-words: stroke,
physical therapy, rehabilitation, human movement, modified constraint-induced
movement therapy.
Resumo
Introdução: O
AVE é uma das doenças mais incidentes no mundo, provocando inúmeras
alterações para a funcionalidade, principalmente relacionadas às funções do
membro superior. Objetivos: Avaliar a
influência da Terapia de Restrição e Indução do Movimento (TRIM) na recuperação
da funcionalidade, amplitude de movimento (ADM) e tônus do membro superior (MS)
de pacientes hemiparéticos crônicos. Material
e métodos: Sete voluntários (52,75 ± 6,63 anos) foram avaliados antes,
imediatamente após e um mês após 12 sessões de TRIM, pela goniometria, Modified Fugl-Meyer Assessment e Modified Ashworth Scale. Resultados: A funcionalidade melhorou
74,7% após intervenção e 79,5% um mês após o término do tratamento. Houve
melhora da movimentação passiva (p = 0,01), da dor (p = 0,004) e da função
motora do MS (p ≤ 0,001); aumento da ADM de flexão, extensão, abdução e
adução do ombro e flexão e desvio radial do punho (p <
0,05) e redução do tônus muscular (p < 0,05). Conclusão: A TRIM foi eficaz para recuperação da ADM do ombro e
punho; retorno da funcionalidade do MS parético e redução da espasticidade,
tendo os resultados se mantido após o término do tratamento.
Palavras-chave: acidente vascular
encefálico, fisioterapia, reabilitação, movimento humano, terapia de restrição
e indução do movimento.
Introduction
In Brazil, the stroke has been considered an impacting condition on
public health due to disabilities and/or functional limitations, standing
between first and third leading cause of morbidity or mortality [1]. Eighty
percent of strokes are caused by cerebral ischemia and twenty percent by
intracerebral or subarachnoid hemorrhage [2].
The stroke is described as an event in which there is an interruption of
the cerebral blood flow, which causes damages in the neurological function,
what are manifested in long term incapacities and an average survival around 1
to 8 years [1].
The major neurological dysfunctions after a stroke are hemiparesis as
well as sensory disorders, which are usually found in the acute phase in the
trunk, upper and lower limbs. However, due to the need of use of the lower
limbs to the gait, the prognosis is better when the brain injury compromises
areas related to the upper limbs, both motor and sensory level.
On the other hand, considering the functional importance of the upper
limb to the activities of daily living (ADLs), it is imperative to explore
treatment strategies for functional recovery of the paretic upper limb [3]. In
addition, epidemiological data indicate that more than 85% of those affected
are deficient in the upper limbs and that of this total, only 25 to 35% reach
functional recovery [3].
In order to achieve an efficient recovery of function, one of the
physical therapy strategies is the stimulation of neural plasticity through
physical exercise, as evidenced by the use of modified Constraint-Induced
Movement Therapy (mCIMT), which encourages the use of
the paretic upper limb with the purpose of minimizing the functional deficits
from multiple brain damages [4].
In this context, the best time to start the rehabilitation is the early
phase of the disease, which corresponds to the first three months after injury,
so as to the functional improvement is more evident at this time and the
recovered motor function may persist due to neural plasticity. Nevertheless, it
is known that in the chronic phase of stroke (six months after) neurological
damages are identified more accurately, since there are compensatory movements
and incorrect relearning or not acquiring of functions, impairing the
functionality [5]. In addition, animal studies have shown that an overload of
stimuli on the paretic limb, as occurs during treatment using mCIMT, in early stages after injury (up to 3 months), may
be detrimental to recovery, as it may stimulate the mechanism of
excitotoxicity, widening the area of the lesion [6]. In this way, therapeutic
care in the early phase after injury is fundamental, so that the paretic limb
presents ideal conditions to respond efficiently to the stimuli applied with
the use of mCIMT in the chronic phase.
The limitation or lack of functionality on the paretic side might be
related to the difficulty of moving the affected limb, encouraging the use of
unaffected limb, in a compensatory way, inducing the development of a behavior
called "learned non-use" [7], which further increases the motor
disability [8].
Studies showed that CIMT promotes the increased use of the affected UL
due to the healthy limb restriction, stimulating the relearning by overcoming
"learned non-use" and inducing a cortical reorganization, through the
repetitive and sustained training, which reverses the loss of the limb cortical
representation, caused by non-use [8,9].
Thus, mCIMT has been a potential method of
sensorimotor gains after a stroke being considered more effective than traditional
therapies to promote changes in the representation of an impaired upper limb in
the cerebral cortex [10].
The aim of this study was to evaluate the influence of modified
Constraint-Induced Movement Therapy (mCIMT) on
functional recovery, range of motion (ROM) and muscle tone of chronic hemiparetic upper limb (UL).
Methods
The study was approved by the Ethics Committee of the Public State
Clinic Hospital Gaspar Viana Foundation (Registration
Number 057/11), and carried out in the Physiotherapy School Clinic, after
signing a consent and information form by the subjects.
It was considered as inclusion criteria: presenting chronic hemiparesis
after a stroke; ages between 50 and 60; ability to actively perform the wrist
flexion movement, metacarpophalangeal and interphalangeal active extension of
10° and wrist extension of 20°; absence of cognitive impairment. The exclusion
criteria were: presenting progressive degenerative disease; stroke recurrence;
deformities and installed and irreversible compensation of the UL; hemiplegic
pattern of the UL; visual and hearing impairment that prevented the
understanding of verbal commands or seeing the task to be performed.
Ten subjects were recruited; three did not complete treatment due to
absence in therapy sessions and stroke recurrence, remaining seven volunteers
for the study.
Evaluation procedures
Modified Fugl-Meyer Motor Assessment
The Modified Fugl-Meyer Assessment (MFMA) is
used to evaluate the motor function of the paretic upper limb [11], considering
7 aspects: Passive movement and pain; Sensitivity; Upper limb motor function;
Coordination / Upper limb velocity; Motor function of lower limb; Coordination
/ Lower limb velocity and Balance. Each item is punctuated as follows: 0 =
cannot be performed; 1 = partially performed and 2 = fully performed, totaling
100 points for normal motor function, 66 being the maximum for the upper limb
and 34 for the lower limb. It should be emphasized that in this study only the
section for upper limbs of the scale was selected.
Modified Ashworth Scale
The Modified Ashworth Scale (MAS) enables the assessment of muscle tone
in individuals affected by stroke, in order to quantify the degree of the
spasticity. Thus the scale is graduated from 0 to 5, where 0 = no increase in
muscle tone; 1 = slight increase in muscle tone, which is manifested by sudden
motion or a minimum resistance at the end of the motion, when the segment is
moved in flexion or extension; 2 = small increase in muscle tone, manifested by
sudden motion followed by minimal resistance throughout the range of motion; 3
= more marked increase in muscle tone throughout most of the range of motion
but the joint is still displaceable; 4 = considerable increase in muscle tone
with difficulty in passive motion; 5 = affected segment is rigid in flexion or
extension [12]. In this study, the scale was used to assess the tone of the
flexor muscle group of the MS.
Goniometry
The ROM was measured in the affected upper limb using manual goniometer
(Carci®) for flexion, extension, adduction,
abduction, medial and lateral rotation of the shoulder; flexion and extension
of the elbow; forearm pronation and supination and flexion, extension, ulnar
deviation and radial wrist.
All evaluations were performed before the physical therapy intervention,
after its end (corresponding to the last day of intervention) and 30 days after
the last intervention session, representing a one-month follow-up.
Intervention procedure
The treatment consisted of applying a modified CIMT protocol three times
a week on alternate days, lasting two hours each session, for a period of four
weeks, totaling 12 sessions. The healthy forearm, wrist and hand were kept in a
neutral position by a splint made for each volunteer. The subjects remained in
sitting position to perform activities with the paretic UL. The material used
to stimulate the paretic limb remained on a table, where also the healthy UL
was supported. The examiner stood opposite the paretic limb, guiding volunteers
to perform the exercises on the ADL board to fit keys, open different locks,
sew on a sewing table, hit a nail.
Thus, in each intervention session, the subjects performed functional
tasks individually, in the following order of execution: grabbing a glass and
bring it to his mouth, grabbing a spoon and taking it up his mouth, combing
hair with a hairbrush, performing activities with assembly games, using the ADL
board, taking a piece of bread or cracker from the plate and taking it up his
mouth and eating it, cleaning the plate and the table with a sponge, bouncing the
ball on the floor, playing the game "Escravos de
Jo" (a traditional Brazilian game which the players have to sing the song
while passing a representative object to the next players on a circle), passing
a sheet of paper between examiners and, finally, greeting them with handshake.
These tasks were repeated throughout the treatment period.
Data analyses
The normality Shapiro-Wilk test was applied to all variables. For
variance analysis and the MFMA and MAS we used the two-way ANOVA test, followed
by the t test; for immediate and delayed pre and post intervention comparisons
and analysis of the goniometer variables among the 14 studied movements, the
Friedman test was applied to compare the three time points of evaluation. The
data was processed in BioEstat software version 5.2.
Results
The volunteers characteristics are described in
Table I, which demonstrates the characterization of the sample regarding age,
chronicity of the lesion, gender and affected body side. In this way, we
observed that the sample shows a larger number of men, with age group
permeating aging, being more affected in the right side of body, and with a
considerable lesion time of 18 ± 3.4 months.
The most significant finding of this study in relation to the ROM refers
to the flexion, extension, abduction and adduction of the shoulder, which
showed significant improvement after the intervention, which was maintained
during follow-up. However, the shoulder rotation movement showed no improvement
in ROM (p > 0.05) and the same occurred with other regions analyzed, as
shown in Table II.
In General MFMA score and its variables, there was an increase in
average passive motion, pain and upper limb motor function after immediate and
delayed treatment when compared to pre-treatment (p = 0.05), already in the
indicators of sensitivity and coordination / speed, the differences were not
significant, as shown in Table III.
Table IV shows the behavior of muscle tone of the flexor muscle group
evaluated by the MAS in the three moments, where progressive reduction of the
muscle tone of the paretic upper limb (p < 0.05) was observed.
Table I - Characterization of the sample.
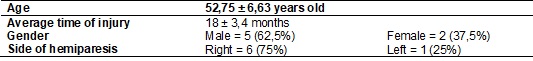
Table II - Mean values and standard deviation of the
degree of motion of the shoulder joints, elbow and wrist and forearm compared
at three time points.
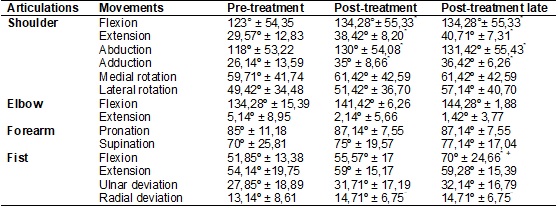
Table III - Mean values and standard deviation of the
paretic upper limb functionality in the three time points.
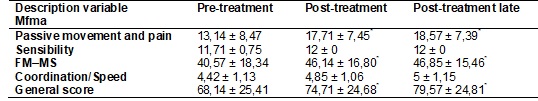
Table IV - Mean values and standard deviation of muscle
tone in the paretic limb in all three time points, as assessed by the Modified
Ashworth Scale (MAS).

Discussion
Although this study presents a reduced sample size, it does not differ
from other studies using mCIMT, since most are
characterized as “case study” [5,13]. However, there
is a general agreement that CIMT enables increase in dexterity and grip
strength, improvement in functional independence, reduced average time to
perform motor tasks [13,14]. However, few studies have
reported effects on a wider sample [15,16] and when the method is applied in
the chronic phase [10,17] and it is evaluated not only the functionality but
also the range of joint motion and the tonus behavior in response to the
intervention, being this the focus of this present study.
From the post-stroke pathophysiological changes, it is possible to
elucidate the effects of mCIMT, since after stroke, a
spontaneous cortical reorganization occurs, which reaches its plateau after 3 months and reflects the
neurotransmission recovery in the spared tissue near or far from the site of
injury [18,19]. From appropriate intervention begins a recovery induced by
training, which is not limited by time, and occurs by synaptic and cytoarquitectural changes and by neurogenesis and can be
observed late and it depends on individual experience and rehabilitation [20].
This way, the cortical reorganization includes increasing of dendrites,
synapses and neurotrophic factors, which are essential for the survival of
nerve cells, characterizing the plasticity of the nervous system. In this
process, after the motor cortex injury, motor homologous regions of the
unaffected hemisphere or the intact cortex adjacent to the injury bear the lost
function. Because of cortical reorganization, which can start from one to two
days after stroke and lasts for months, it is possible to recover, at least in
part, the skills, which had been lost [21].
Although a direct measure of plasticity was not performed in this study
to demonstrate the effects of mCIMT on a possible
cortical reorganization, indirect measures (ROM, motor function and muscle
tone) showed the reflex of the plastic alterations induced by the mCIMT.
The results of this study regarding the paretic shoulder range of motion
and radial deviation of the wrist, which were maintained 30 days after the end
of treatment, suggest that the re-learned movements were incorporated into the
volunteers daily life activities, reaffirming the efficacy of the intervention
and its role in a possible cortical reorganization, since the mCIMT is characterized as intensive and incorporates
repetitive activities and progressive difficulty, being proven its effects in
the short and long term [22].
Modified CIMT is known to stimulate neuroplasticity, as it promotes
increased cortical excitability, metabolic rate and blood flow in the brain,
allowing an increase in the sensory and motor areas of cortical representation,
both contralateral and ipsilateral to the affected upper limb, as well as
bilaterally in the hippocampus, contributing to the functional recovery of the
affected limb [23].
It is important to highlight that mCIMT shows
effectiveness in motor recovery from months to years after the injury [24],
therefore contemplating the rehabilitation needs of the subjects in this study.
In the context of chronicity, it is known that after stroke the tendon
and muscular resistance to speed-dependent stretching is remarkable, which characterizes
the upper motor neuron injury [25,26]. Despite the
spasticity impacts after a stroke are still questionable, although the
treatments for abnormal tone control show good results, they did not correlate
with the improvement in functionality [27]. There is no doubt that the control
of spasticity facilitates increasing ROM opposing to postural pattern shown in
hemiparesis, since the muscles committed are properly stimulated, as it occurs
during mCIMT treatment. Thus, we consider that the
increase in ROM and improvement in muscle tone control observed in this study
are intrinsically associated.
Thus, the control of spasticity in this study seems to have resulted
from inherent factors to the method of intervention chosen and the protocol
executed, since the proposed activities stimulated motion of the extensor
muscles of the UL and abductors in order to inhibit the predominant flexor and
adductor pattern. With repetitive and intense stimulation during application of
mCIMT, there was an activation of the physiological
mechanism of reciprocal inhibition, compromised by injury. As the proposed
tasks were antagonistic to the postural pattern shown by the UL, it is
considered that the reciprocal inhibition process has been activated,
facilitating the inhibition of abnormal muscle tone and also the functional
recovery [28-30].
Considering the results found in this study, both the ROM and control of
muscle tone contributed to the improvement of functionality. In this study, the
overall score of MFMA showed clear recovery immediately after the intervention,
which was held 30 days after its completion.
Improved functionality in this study from the application of MFMA scale
was 74.1% in the post-treatment immediately and 79.5% in the late post-treatment
compared to pre-treatment values. This improvement can be attributed to the
intense repetition of functional activities. According to Souza et al. [31], they stimulate motor
learning, providing better handling of the objects.
Cortical reorganization in patients with chronic stroke who underwent mCIMT has been reported by Cruz, Santana and Dumas [7],
which related neuroimaging with functional improvement and reaffirmed the
possibility of learning and motor cortical reorganization even when the
intervention is applied in the chronic phase. They justify that intensive motor
training promotes brain development, possibly leading to the recruitment of a
large number of neurons adjacent to the injury to the innervation of the UL
paretic muscles, inducing neuronal plasticity, which can generate neuroplasmatic modeling in motor areas.
In this study, even if there has not been performed neuroimaging, the
results of ROM, muscle tone and functional recovery suggest the presence of
cortical reorganization when using mCIMT later after
the injury.
Conclusion
The mCIMT protocol used in this study was
effective for the improvement of the range of motion, stabilization of the
muscle tone and recovery of the functionality of the upper limb of chronic hemiparetic patients after stroke, and the results were
maintained until one month after the intervention, possibly by the
incorporation of the motor tasks re-learned to the activities of daily life.
However, there is a need for studies with a greater number of volunteers
with this profile in the region, and that can be followed up in longer or even
evaluated interventions in a longer posttreatment period.
References
- Monteiro KS, Souza
CG, Franco CIF, Moura JV. Caracterização funcional de indivíduos acometidos por
acidente vascular encefálico assistidos em uma unidade
de terapia intensiva. Rev Bras Cienc Saude 2013(17):269-74.
- Fonseca LHO, Rosa
MLG, Silva AC, Maciel RM, Volschan A, Mesquita ET. Análise das barreiras à
utilização de trombolíticos em casos de acidente vascular cerebral isquêmico em
um hospital privado do Rio de Janeiro, Brasil. Cad Saúde Pública 2013;29(12):2487-96.
- Lang CE, Bland MD, Bailey RR, Schaefer SY, Birkenmeier
RL. Assessment of upper extremity impairment, function, and activity following
stroke: foundations for clinical decision making. J Hand Ther 2013;26(2):104-115.
- Bueno GDP, Lúcio AC,
Oberg TD, Cacho EWA. Terapia de restrição e indução modificada do movimento em
pacientes hemiparéticos crônicos: Um estudo piloto. Fisioter Mov 2008;21(3):37-44.
- Rodrigues FZ, Marinho
GKA, Silva AT, Silva AM, Sales EV, Mariano KOP. Terapia de Restrição e Indução
ao Movimento no membro superior parético crônico-relato de caso. Rev Neurocienc 2013;21(4):568-73.
- Kozlowski, DA, James, DC, Timothy S. Use-dependent exaggeration of
neuronal injury after unilateral sensorimotor cortex lesions. J
Neurosci 1996;16(15):4776-86.
- Cruz PC, Santana LA,
Dumas FLV. Fisioterapia e neuroplasticidade após acidente vascular encefálico:
uma revisão sistemática. Universitas: Ciência & Saúde 2012;10(1):61-78.
- Garcia JM, Knabben
RJ, Pereira ND, Ovando AC. Terapia por Contensão Induzida (TCI) em adolescentes
com hemiparesia espástica: relato de caso. Fisioter Mov 2012;25(4):895-906.
- Zilli F, Lima ECBA,
Kohler MC. Neuroplasticidade na reabilitação de pacientes acometidos por AVC
espástico. Rev Ter Ocup Univ 2014;25(3):317-22.
- Palavro BEM, Schuster
RC. Efeitos da terapia de contenção induzida adaptada na funcionalidade e
qualidade de vida de pacientes hemiparéticos. Rev Fisioter S Fun 2013;2(2):51-60.
- Sousa RCP, Terra FR,
Carbonero FC, Campos D. Terapia de restrição e indução do movimento em
hemiparéticos. Rev Neurocienc
2012;20(4):604-11.
- Gregson JM, Leathley M, Moore P, Sharma AK,
Smith TL, Watkins CL. Reliability of the tone assessment scale and the Modified
Ashworth Scale as clinical tools for assessing poststroke
spasticity. Arch
Phys Med Rehabil 1999;80(9):1013-6.
- Meneghetti CHZ, Silva
JÁ, Guedes CAV. Terapia de restrição e indução ao movimento no paciente com
AVC. Rev Neurocienc 2010;18(1):18-23.
- Areeat S, Nijasri
CS. Effectiveness of constraint-induced motion therapy in chronic stroke
patients. J Med Assoc Thailand 2004;87:148-55.
- Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G.
Remodeling the brain: plastic structural brain changes produced by different
motor therapies after stroke. Stroke 2008;39(5):1520-5.
- Wu C, Chen C. Kinematic and clinical analyses of upper-extremity motions
after constraint-induced motion therapy in patients with stroke: a randomized
controlled trial. Arch Phys Med Rehabil 2007;88(8):964-70.
- Magalhães JP, Letiere M, Silva AT, Kosour C,
Reis LM. Efeito
da terapia de restrição e indução ao movimento em pacientes hemiparéticos
crônicos pós-AVC. Rev Neurocienc
2013;21(3):333-8.
- Carey LM, Seitz RJ. Functional neuroimaging in stroke recovery and
neurorehabilitation: Conceptual issues and perspectives. Int
J Stroke 2001;2:245-264.
- Dobkin BH. Clinical
Practice. Rehabilitation after Stroke. N Engl J Med 2001;352:1677-84.
- Chen H, Epstein J, Stern E. Neural plasticity after acquired brain
injury: Evidence from functional neuroimaging. PM&R 2010; Suppl.2:S306-S312.
- Souza CAB, Aquino
FAO, Barbosa MLC, Alvarez RBP, Turienzo TT. Influência da neuroplasticidade no
controle motor. Rev Unilus Ens Pesq 2013;10(19):6-11.
- Lima RCM, Nascimento
LR, Michaelsen ST, Polese JC, Pereira ND, Teixeira-Salmela LF. Influences of hand dominance on the maintenance of
benefits after home-based modified constraint-induced motion therapy in
individuals with stroke. Braz J Phys Ther 2014;18(5):435-44.
- Gamba RT, Cruz DMC.
Efeitos da Terapia por Contenção Induzida em longo prazo em pacientes pós-AVC. Rev Neurocienc 2011;19(4):735-740.
- Hodics T, Cohen LG. Functional
neuroimaging in motor recovery after stroke. Top Stroke Rehab 2005;12(2):15-21.
- Sheean G. The
pathophysiology of spasticity. Eur J Neurol 2002;9(Suppl.1):3-9.
- Dietz V, Sinkjaer T. Spastic motion disorder:
impaired reflex function and altered muscle mechanics. Lancet Neurol 2007;6:725-33.
- Bhakta BB. Management of spasticity in stroke.
Brit Med Bull 2000;56(2):476-85.
- Pompeu JE, Mattos
ECT, Kohn AF. Avaliação da inibição recíproca em humanos durante contrações isométricas dos músculos tibial anterior e sóleo. Fisioter Pesqui 2009;16(3):258-62.
- Chakravarty MA. Spasticity mechanisms – for the
clinician. Spinal Cord 2010;1(149):1-10.
- Bhagchandani N, Schindler-Ivens S. Reciprocal inhibition post-stroke is related to
reflex excitability and motion ability. Clin Neurophysiol 2012;123:2239-46.
- Sousa RCP, Terra FR,
Carbonero F, Campos D. Terapia de restrição e indução do movimento em
hemiparéticos. Rev Neurocienc 2012;20(4):604-11.