REVIEW
Effect
of incentive spirometry after cardiac surgery: protocol for a systematic review
Efeito da espirometria
de incentivo no pós-operatório de cirurgia cardíaca: protocolo para uma revisão
sistemática
Elinaldo da Conceição dos
Santos1,2, PhD, PT, Ana Carolina Pereira Nunes Pinto 2,3,
M.Sc., PT, Juliana Ribeiro Fonseca Franco de Macedo4,
M.Sc., PT, Adriana Claudia Lunardi1,5,
PhD, PT
1Master’s and Doctoral
Programs in Physical Therapy of Universidade Cidade de São Paulo, São Paulo, Brazil, 2Department
of Biological and Health Sciences. Universidade
Federal do Amapá, Macapá,
Brazil, 3Department of Physical Therapy. University of Pittsburgh,
United States. Fulbright Program, 4Department of Health Sciences.
Catholic University of Louvain, Belgium, 5Department of Physical
Therapy of School of Medicine of Universidade de São
Paulo, São Paulo, Brazil
Received: December
4, 2019; accepted: January
15, 2020.
Correspondence: Prof. Elinaldo da Conceição dos Santos, Universidade Federal do
Amapá, Departamento de Ciências Biológicas e da Saúde, Rod. Juscelino
Kubitschek, km 02 Jardim Marco Zero 68903-419 Macapá AP
Elinaldo da Conceição dos
Santos: drelinaldo@yahoo.com.br
Ana Carolina Pereira
Nunes Pinto: anacarolinapnp@hotmail.com
Juliana Ribeiro Fonseca
Franco de Macedo: demacedo.franco@gmail.com
Adriana Claudia Lunardi: adriana.lunardi@unicid.edu.br
Abstract
Introduction: Patients receiving cardiac surgeries present high risk of developing
postoperative complications. Incentive spirometry (IS) is used for the
prevention and treatment of postoperative pulmonary complications in patients
undergoing cardiac surgeries. Publications have suggested that IS is ineffective. In contrast, some studies have shown that
when IS is adequately used, it may lead to beneficial
outcomes. Objectives: To assess the effect of IS in patients undergoing
cardiac surgeries. Methods/design: Systematic Reviews with randomised and quasi-randomised
trials with adult patients undergoing cardiac surgeries, evaluating the effect
of flow or volume-oriented IS. Outcome measures: postoperative pulmonary
complications; adverse events; mortality; length of hospital stay; length of
intensive care unit stay; reintubation rate; pulmonary function; antibiotic
use; oxygenation; and respiratory muscle strength. Search: MEDLINE,
EMBASE, CENTRAL, PEDro, CINAHL, LILACS, SCIELO,
Allied, AMED, Scopus, Open Grey database, the World Health Organization
International Clinical Trials Registry Platform (WHO ICTRP),
ClinicalTrials.gov, clinicaltrialsregister.eu, and ReBec.
Two authors will independently extract data. PEDro
scale will be used to evaluate the methodological quality of the studies.
Meta-analysis will be performed using the inverse variance method and the
random effects model in RevMan 5.3. We will use the I2
statistic to estimate the amount of heterogeneity across studies in each
meta-analysis. Ethics and dissemination: The approval of an ethical
committee is not required. Only clinical trials that have complied with ethical
guidelines and followed the Declaration of Helsinki, will be included in this
systematic review. The findings of this study will help clarify
uncertainties about the effects of incentive spirometry in the postoperative
period of cardiac surgery and may be disseminated to clinicians, assisting in
decision making and including the best evidence in the treatment of their
patients. Discussion: This review will clarify the uncertainty over
whether IS is a useful technique for patients
undergoing cardiac surgeries. While good quality studies have shown IS is an effective prophylactic technique, other studies have
suggested that there is no evidence to support IS utilization.
Keywords: Incentive spirometry; Cardiac Surgery; Postoperative; Systematic
Review.
Resumo
Introdução: Pacientes submetidos
a cirurgias cardíacas apresentam alto risco de desenvolver complicações
pós-operatórias. A espirometria de incentivo (EI) é utilizada para a prevenção
e tratamento de complicações pulmonares pós-operatórias em pacientes submetidos
a cirurgias cardíacas. As publicações têm sugerido que a EI é inefetiva. Em
contrapartida, alguns estudos têm demonstrado que quando a EI é utilizada
adequadamente, pode levar a resultados benéficos. Objetivos: Avaliar o
efeito da EI em pacientes submetidos a cirurgias cardíacas. Métodos/desenho:
Revisões sistemática de estudos randomizados e quase randomizados com pacientes
adultos submetidos a cirurgias cardíacas, avaliando o efeito da EI a fluxo ou a
volume. Medidas de desfecho: complicações pulmonares pós-operatórias; eventos
adversos; mortalidade; tempo de internação hospitalar; tempo de internação na
unidade de terapia intensiva; taxa de reintubação;
função pulmonar; uso de antibióticos; oxigenação e força muscular respiratória.
Busca: MEDLINE, EMBASE, CENTRAL, PEDro, CINAHL,
LILACS, SCIELO, Allied, AMED, Scopus, Open Grey database, World Health
Organization International Clinical Trials Registry Platform (WHO ICTRP),
ClinicalTrials.gov, clinicaltrialsregister.eu, e ReBec.
Dois
autores irão extrair dados de forma independente. A escala PEDro
será utilizada para avaliar a qualidade metodológica dos estudos. A
meta-análise será realizada utilizando o método do inverso da variância e o
modelo de efeitos aleatórios no RevMan 5.3. Será
utilizada a estatística I2 para estimar a heterogeneidade entre os
estudos em cada meta-análise. Ética e disseminação: A aprovação de um
comitê de ética não é necessária. Somente estudos clínicos que tenham cumprido
as diretrizes éticas e seguido a Declaração de Helsinque serão incluídos nesta
revisão sistemática. Os resultados deste estudo ajudarão a esclarecer
incertezas sobre os efeitos da espirometria de incentivo no período
pós-operatório de cirurgia cardíaca e poderão ser divulgados aos clínicos,
auxiliando na tomada de decisões e incluindo as melhores evidências no
tratamento de seus pacientes. Discussão: Esta revisão esclarecerá a
incerteza sobre a utilidade da EI para pacientes submetidos à cirurgia
cardíaca. Embora estudos de boa qualidade tenham demonstrado que a EI é uma
técnica profilática eficaz, outros estudos sugeriram que não há evidências que
apoiem a utilização da EI.
Palavras-chave: espirometria de
incentivo, cirurgia cardíaca, pós-operatório, revisão sistemática.
Introduction
Worldwide, nearly two million patients undergo cardiac surgeries anuually [1]. The patients receiving cardiac surgeries
present high risk of developing postoperative complications [2]. Mortality
rates may vary from 2.94% [3] to 6.50% [4]. The postoperative time, the type of
cardiac surgery and the type of care received by the patients during the
postoperative period are factors that may influence the mortality rates [5,6].
The costs of cardiac surgeries are high [5,6] and may increase when the
patients receive low quality healthcare [7].
Incentive spirometry (IS) is a low-cost technique used for the
prevention and treatment of postoperative pulmonary complications in patients
undergoing cardiac surgeries [8]. IS comprises the use of volume or flow
oriented devices, designed to provide visual feedback and to stimulate deep,
slow and sustained inspirations [9]. Studies evaluating the effectiveness of IS
have shown conflicting results. Several publications have suggested that IS is ineffective [8,10]. Nevertheless, nearly 42% of
physiotherapists keep using IS after thoracic surgical procedures [11]. In
contrast, some studies have shown that when IS is
adequately used, it may lead to beneficial outcomes [12,13]. These studies have
suggested that IS may improve blood arterial gas parameters14 and prevent and
treat atelectasis [13]. Further well-conducted studies are needed to guide
clinical decision making and avoid ineffective practices [15].
To our knowledge, there are no systematic reviews exclusively evaluating
the effect of IS in patients undergoing cardiac surgeries, with extensive
search of databases and no language or time of publication limitations. Thus,
we designed this systematic review to answer the question: - Is IS safe and more effective than sham IS, nothing, or other
therapies for reducing postoperative pulmonary complications, mortality, length
of hospital and/or intensive care unit (ICU) stay, reintubation rate, pulmonary
function, respiratory muscle strength, oxygenation and antibiotic use in
patients undergoing cardiac surgery?
Methods/Design
Design
We will perform a systematic review following the recommendations proposed
by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis
(PRISMA) [16]. This review protocol will be prospectively registered in the
International Prospective Register of Systematic Reviews (PROSPERO)
Eligibility
criteria
Types
of studies
Randomized and quasi-randomized trials will be included in this
systematic review. We will consider that quasi-randomized study are controlled
trials in which participants are allocated to different groups using a not trully random method of allocation (eg,
medical record number, or date of birth) [17].
Types
of participants
Trials including patients aged 18 years and over undergoing cardiac
surgeries.
Types
of interventions
We will include trials evaluating the effect of postoperative flow or volume-oriented IS [18] in patients who have undergone
cardiac surgeries. The IS may have been used alone, or combined with other
techniques, after cardiac surgeries. Comparison groups can be: nothing, sham
IS, other therapies or other comparisons in which IS effect can be exclusively
evaluated, such as: IS + usual care versus usual care alone; IS + oxygen
therapy versus oxygen therapy alone; IS + continuous positive airway pressure
(CPAP) versus CPAP alone; and so on.
Outcome
measures
-
Primary outcomes
- Postoperative
pulmonary complications, including independent analysis of the outcomes:
- Atelectasis:
radiologic, bronchoscopic or clinical diagnosis.
- Respiratory
infection (pneumonia): radiologic or clinical diagnosis.
- Adverse
events: any reaction, harm, or complication associated with IS reported in the
included studies.
- Mortality:
any mortality cause will be accepted.
-
Secondary outcomes
- Length
of hospital stay: the number of days spent in hospital after the cardiac
surgical procedure will be registered.
- Length
of intensive care unit stay: the number of days spent in ICU after the cardiac
surgical procedure will be registered.
- Reintubation
rate: the number of intubation events following planned extubation
will be registered.
- Pulmonary
function: all pulmonary function variables will be accepted.
- Antibiotic
use: the number of antibiotics and the number of days using antibiotics will be
registered.
- Oxygenation:
arterial oxygen partial pressure (PaO2), oxygenation index (OI), and
peripheric and central arterial oxygen saturation (SaO2) will be
accepted.
- Respiratory
muscle strength: Maximal inspiratory and expiratory pressures measured with
digital or analog manovacuometer or manometer will be
accepted.
Report
characteristics
We will include studies performed in any year. No language restrictions
will be used in the selection. We will also include grey literature data.
Data
Sources and Searches
We will search MEDLINE, EMBASE, Cochrane Central Register of Controlled
Trials (CENTRAL), PEDro, CINAHL, LILACS, SCIELO,
Allied and Complementary Medicine Database (AMED) and Scopus using relevant
descriptors and synonyms, adapting the search to the requirements of each
database.
We will also search the Open Grey database, the World Health
Organization International Clinical Trials Registry Platform (WHO ICTRP),
ClinicalTrials.gov, clinicaltrialsregister.eu, and ReBec
(Brazilian Register of Clinical Trials) to identify published, ongoing, and
unpublished studies. We will hand search abstracts from scientific meetings,
including the International Conference on Cardiovascular and Thoracic surgery,
International Conference on Cardiovascular Medicine and Cardiac Surgery,
International Symposium of Cardiopulmonary Physiotherapy and Intensive Care
Physiotherapy, European Society of Cardiology Congress, and European
Association for Cardio-Thoracic Surgery Annual Meeting. We will contact authors
of relevant studies to identify additional studies. Finally, we will use the
technique of snowballing, searching the lists of references of the included
studies.
Search
strategy
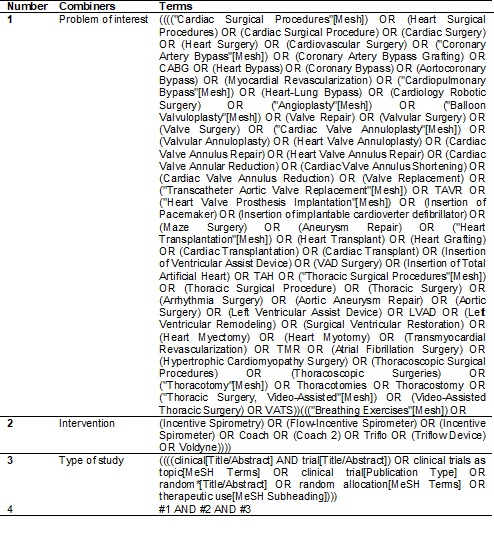
We will use the terms related to the problem of interest and to the
therapeutic technique. The terms are described in Table I.
Table
I - Systematic review Search Strategy.
Study selection
Three authors will independently select the studies for inclusion in
this review (ECS, JRFFM e ACPNP). Two authors will select potential studies
identified by the search strategy based on the pre-specified eligibility
criteria. First, duplicated studies (found in more than one database) will be
excluded. When duplicated reports are found (studies with the same
participants, with the same outcome measurements and using the same time points
for the assessments), the report with the smaller sample size will be excluded.
If reports with the same participants but different outcome measurements or
using different time points for the assessments are found, both the reports
will be included (the two reports will be considered as parts of only one study).
Second, the authors will read the study titles and abstracts; and, if
necessary, they will finally read the full texts. Studies that do not match the
inclusion criteria for this review will be excluded. The reasons for exclusion
of the studies that are fully read will be presented. Disagreements between
authors regarding study inclusion will be resolved by the third author (ACPNP).
We will use Rayyan app [19] to optimize the process of screening and selection
of studies. The flow chart of this systematic review is shown in figure 1.
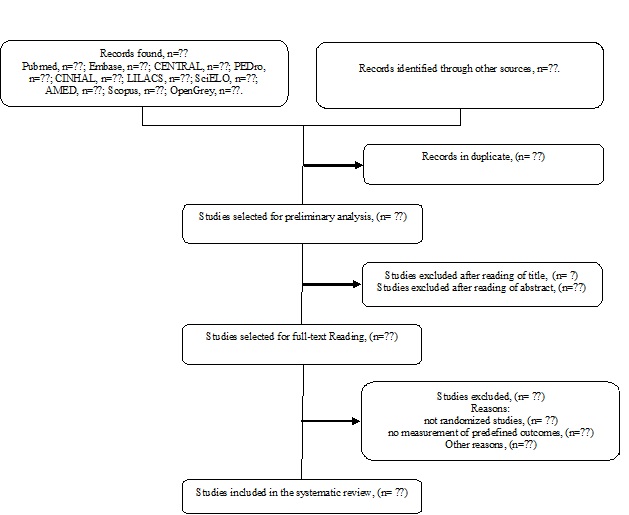
Figure
1 - Flow chart of systematic review.
Data
Extraction and management
Data will be managed and stored on Dropbox. Two authors (ECS e JRFFM)
will independently extract data. Discrepancies or disagreements will be solved
by a third author (ACPNP). The authors of the relevant studies will be
contacted in the case of missing study details. We will use a predefined form
to extract data from included studies. The form will have information related
to: - the patients (demographic and clinical characteristics); - the surgery
(the type of surgery: elective, urgency, or emergency; surgery duration; type
of surgical incision; use of extracorporeal circulation); - intervention
characteristics (time of the intervention commencing, type of device, training
details: volume, frequency, recovery, duration, use of co-interventions, adherence
to training); - time points used for the assessments; - number of patients lost
to follow up (in each group); - reasons for loss to follow up; - approach for
handling missing data (data imputation/how data imputation was performed, use
of intention-to-treat approach); - sources of funding; - possibility of
conflict of interests; - adverse events; - outcome measures; - protocol
deviations.
To
access the feasibility of performing a meta-analysis, we will also extract the
following data for each primary and secondary outcome measure: - total number
of patients (in each group); - number of events in each group (for dichotomous
outcomes); - mean, standard deviation, standard error, median, interquartile
range, minimum, maximum, 95% confidence interval (CI) (for continuous
outcomes); - p value.
Assessment
of Methodological Quality in included studies and quality of the body of
evidence
We will assess the methodological characteristics of included studies
with PEDro [20] scale, as it is reliable [20], acceptable
among assessors [20], and presents strong correlation with Cochrane Risk of
Bias Tool [21]. We will evaluate the quality of evidence using the Grading of
Recommendations Assessment, Development and Evaluation (GRADE) [22]. GRADE is a
simple and integral approach that guides the judgement on the quality of the
body of evidence. GRADE judgement is based on the overall risk of bias,
consistency of the results, directness of the evidence, publication bias and
precision of the results for each outcome. The GRADE profiler software,
available online, will be used to summarize our findings on the quality of
evidence [23]. Assessment of risk of bias, and assessment of the quality of
evidence will be performed by two previously trained review authors (ECS and
JRFFM) independently. All the disagreements in the assessment of the risk of
bias or quality of evidence will be solved through discussion or, if required,
by consulting with a third author (ACPNP).
Data
synthesis and analysis
When at least two studies are sufficiently homogeneous in terms of
participants, interventions and outcome measurements, we will pool their
results into meta-analysis. When insufficient data is presented in the primary
studies to enter into meta-analysis, study authors will be contacted to request
access to the missing data.
Meta-analysis will be performed using the inverse variance method and
the random effects model in Review Manager version 5.3 [24]. Continuous
variables will be analyzed using the weighted mean difference with 95% CI.
Dichotomous variables will be analyzed using relative risk (RR) with 95% CI.
Dealing
with missing data
If a trial does not provide the standard deviation and after contacting
the authors, they do not provide this measure, we will impute it using data
from another trial which has evaluated the same outcome at an identical
follow-up time point. This method is recommended in the Cochrane Handbook for
Systematic Reviews of Interventions [17]. If insufficient data is provided
following contact with the author, the results of the trial will be summarized
only in qualitative synthesis.
Assessment
of heterogeneity
We will use the I2 statistic to estimate the amount of
heterogeneity across studies in each meta-analysis. As suggested in Cochrane
Handbook for Systematic Reviews of Interventions, if heterogeneity is
substantial (I2 ≥ 50%), a subgroup or sensitivity analysis
will be considered [17]. These analyses will involve the exclusion of one
pre-determined study (subgroup) from the meta-analysis. The I2 of
the remaining studies in the meta-analysis will be calculated to investigate if
the excluded study was one potential source of heterogeneity. We will consider
the following subgroups when investigating their effect on heterogeneity: age,
sex, body mass index, type of surgery, utilization of extracorporeal
circulation: severity of the disease, details of intervention, such as the use
of different types of devices, frequency, duration, and time of the
intervention commencing. We will consider the following information for
sensitivity analysis: no blinding or inappropriate blinding of outcome
assessors, inappropriate randomization methods, large number (>20%) of
patients lost to follow up, imputation of standard deviation or when adherence
is not reported.
Assessment
of reporting biases
When at least 10 studies are included in a meta-analysis we will explore
the likelihood of reporting biases visually inspecting funnel plots. For
continuous outcomes, Egger’s test will be used to detect possible small study
bias as recommended in Cochrane Handbook for Systematic Reviews of
Interventions [17].
Discussion
This systematic review aims to assess the effect of IS in patients
undergoing cardiac surgeries. To ensure that this systematic review of
interventions is of high quality, we will follow Cochrane Handbook of
Systematic Reviews recommendations [17]. We believe this scientifically
rigorous review with transparent methods will provide a deep critical appraisal
on the current evidence. While some studies have shown IS is
an effective prophylactic technique [12-14], other studies have suggested that
IS is only as effective as cough and deep breathing
regimens [25], and that there is no evidence to support its utilization in clinical
practice [8,10]. Our review will clarify the uncertainty over whether this
widely used technique is useful for patients undergoing cardiac surgeries.
Limitations
and strengths
Limitations
This study aims to complete a comprehensive systematic review on the
effect of IS in patients who have undergone cardiac surgeries, and if possible,
to pool data into meta-analysis for reducing the probability of type 2 error in
the comparisons. Potential limitations for this study include the possibility
of finding: biased studies, such as those with lack of blindness of outcome
assessors, or without proper randomization; substantial heterogeneity across
studies which make them unsuitable for clustering or meta-analysis: or small
sample studies that do not allow us to provide precise estimates of the
effects.
Strengths
We believe that the strengths of this systematic review include the
transparency, the strict methods, the evaluation of the quality of evidence for
each outcome and the extensive and careful searches, without language or date
of publication restrictions. We will be able to identify grey literature data
and ongoing studies, to include important updated supplementary insights on
this topic and to perform rigorous critical appraisal on the current body of
evidence. Furthermore, we will include patients undergoing only cardiac
surgeries. We anticipate this will provide more clinical homogeneity than in
previous reviews [8,10,15].
Reporting
standards
This systematic review protocol was written as per the PRISMA-P
guidelines [26].
References
- Parikh
CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z et al. Postoperative biomarkers predict
acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011;22(9):1748-57.
https://doi.org/10.1681/asn.2010121302
- Ball
L, Costantino F, Pelosi P. Postoperative complications of patients undergoing
cardiac surgery. Curr Opin Crit Care 2016;22(4):386-92. https://doi.org/10.1097/mcc.0000000000000319
- Siregar S, Groenwold
RH, de Mol BA, Speekenbrink RG, Versteegh
MI, Brandon Bravo Bruinsma GJ et al. Evaluation of
cardiac surgery mortality rates: 30-day mortality or longer follow-up? Eur J Cardiothorac Surg 2013;44(5):875-83.
https://doi.org/10.1093/ejcts/ezt119
- Hansen
LS, Hjortdal VE, Andreasen JJ, Mortensen PE, Jakobsen
CJ. 30-day mortality after coronary artery bypass grafting and valve surgery
has greatly improved over the last decade, but the 1-year mortality remains
constant. Ann Card Anaesth 2015;18(2):138-42.
https://doi.org/10.4103/0971-9784.154462
- Gelsomino S, Lorusso
R, Livi U, Masullo G, Lucà F, Maessen J et al. Cost and
cost-effectiveness of cardiac surgery in elderly patients. J Thorac Cardiovasc Surg 2011;142(5):1062-73.
https://doi.org/10.1016/j.jtcvs.2011.02.013
- Leyva
F, Qiu T, Evison F, Christoforou C, McNulty D, Ludman
P et al. Clinical outcomes and costs of cardiac revascularisation
in England and New York state. Open Heart 2018;5(1):e000704.
https://doi.org/10.1136/openhrt-2017-000704
- Birkmeyer JD, Gust C, Dimick JB, Birkmeyer NJ, Skinner
JS. Hospital quality and the cost of inpatient surgery in the United States.
Ann Surg 2012;255(1):1-5. https://doi.org/10.1097/sla.0b013e3182402c17
- Overend TJ, Anderson CM, Lucy
SD, Bhatia C, Jonsson BI, Timmermans C. The effect of incentive spirometry on
postoperative pulmonary complications: a systematic review. Chest
2001;120(3):971-8. https://doi.org/10.1378/chest.120.3.971
- Restrepo
RD, Wettstein R, Wittnebel
L, Tracy M. Incentive spirometry: 2011. Respir Care 2011;56(10):1600-4.
https://doi.org/10.4187/respcare.01471
- Carvalho
CR, Paisani DM, Lunardi AC. Incentive spirometry in
major surgeries: a systematic review. Rev Bras
Fisioter 2011;15(5):343-50.
https://doi.org/10.1590/s1413-35552011005000025
- Santos EC, Silva JS,
Assis Filho MTT, Vidal MB, Lunardi AC. Use of lung expansion techniques on drained and non-drained pleural
effusion: survey with 232 physiotherapists. Fisioter
Mov 2020;33:1-10. https://doi.org/10.1590/1980-5918.33.ao05
- Eltorai AEM, Baird GL, Eltorai AS, Healey TT, Agarwal S, Ventetuolo
CE et al. Effect of an incentive spirometer patient reminder after coronary
artery bypass grafting: a randomized clinical trial. JAMA Surg 2019;154(7):579-88.
https://doi.org/10.1001/jamasurg.2019.0520
- Iverson
LI, Ecker RR, Fox HE, May IA. A comparative study of IPPB, the incentive
spirometer, and blow bottles: the prevention of atelectasis following cardiac
surgery. Ann Thorac Surg 1978;25(3):197-200.
https://doi.org/10.1016/s0003-4975(10)63521-7
- Yazdannik A, Bollbanabad
HM, Mirmohammadsadeghi M, Khalifezade
A. The effect of incentive spirometry on arterial blood gases after coronary
artery bypass surgery (CABG). Iran J Nurs Midwifery
Res 2016;21(1):89-92. https://doi.org/10.4103/1735-9066.174761
- Narayanan
ALT, Hamid SRGS, Supriyanto E. Evidence regarding
patient compliance with incentive spirometry interventions after cardiac,
thoracic and abdominal surgeries: A systematic literature review. Can J Respir Ther 2016;52(1):17-26.
- Moher
D, Liberati A, Tetzlaff J,
Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and
meta-analyses: the PRISMA statement. PLoS Med
2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097
- Higgins
JPT, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions. 2nd
ed. Chichester UK: The Cochrane Collaboration and John Wiley & Sons; 2019.
703 p. https://doi.org/10.1002/9781119536604
- Eltorai AEM, Szabo AL, Antoci V Jr, Ventetuolo CE, Elias
JA, Daniels AH et al. Clinical effectiveness of incentive spirometry for the
prevention of postoperative pulmonary complications. Respir Care
2018;63(3):347-52. https://doi.org/10.4187/respcare.05679
- Ouzzani M, Hammady
H, Fedorowicz Z, Elmagarmid
A. Rayyan – a web and mobile app for systematic review. Syst Rev 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4
- Maher
CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled
trials. Phys Ther 2003;83(8):713-21.
https://doi.org/10.1093/ptj/83.8.713
- Yamato
TP, Maher C, Koes B, Moseley A. The PEDro scale had acceptably high convergent validity,
construct validity, and interrater reliability in evaluating methodological
quality of pharmaceutical trials. J Clin Epidemiol 2017;86:176-181.
https://doi.org/10.1016/j.jclinepi.2017.03.002
- GRADE
Working Group. Grading quality of evidence and strength of recommendations. BMJ
2004;328(7454):1490. https://doi.org/10.1136/bmj.a139
- GRADEpro GDT 2015. Grade’s
software for summary of findings tables, health technology assessment and
guidelines. https://gradepro.org
- Review
Manager 5 (RevMan 5) [Computer program]. Version 5.3. Copenhagen: Nordic Cochrane Centre, Cochrane; 2014.
- Rupp
M, Miley H, Russell-Babin K. Incentive spirometry in
postoperative abdominal/thoracic surgery patients. AACN Adv Crit
Care 2013;24(3):255-63. https://doi.org/10.4037/nci.0b013e31828c8878
- Moher
D, Shamseer L, Clarke M, Ghersi
D, Liberati A, Petticrew M,
Shekelle P, Stewart LA. Preferred Reporting Items for
Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev
2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1