Fisioter Bras 2022;23(3):357-71
ORIGINAL ARTICLE
Effect of cardiac rehabilitation on the nociceptive
threshold increased of hypertensive subjects
Efeito
da reabilitação cardíaca sobre o limiar nociceptivo aumentado de indivíduos
hipertensos
Ana
Clara Desiderio Maldonado*, João Paulo Prado*, Tarcísio Nema
de Aquino*, Fernanda de Carvalho Vidigal**, Juliana Bassalobre
Carvalho Borges***, Giovane Galdino de Souza***
*Instituto
das Ciências da Motricidade, Universidade Federal de Alfenas, Alfenas, MG,
**Curso de Nutrição, Universidade Federal de Alfenas, Alfenas, MG, ***Professor
do Curso de Fisioterapia, Universidade Federal de Alfenas, Alfenas, MG
Received: May 21, 2021; Accepted:
March 25, 2022.
Correspondence: João Paulo Prado, Universidade Federal
de Alfenas, Unidade Educcional II, Alfenas, Av.
Jovino Fernandes Sales, 2600, 37133-840 Alfenas MG, E-mail:
joaopauloprado51@gmail.com
Ana
Clara Desiderio Maldonado: anadesimald@gmail.com
João
Paulo Prado: joaopauloprado51@gmail.com
Tarcísio
Nema de Aquino: tarcisioaquino@gmail.com
Fernanda
de Carvalho Vidigal: fcvidigal@gmail.com
Juliana
Bassalobre Carvalho Borges:
juliana.borges@unifal-mg.edu.br
Giovane
Galdino de Souza: giovanegsouza@yahoo.com.br
Abstract
Objectives: Hypertensive
subjects have an increased nociceptive threshold, which may contribute to the
pain perception reduction. Thus, the aim of this study was to evaluate the
effect of a cardiac rehabilitation program (CRP) on the increased nociceptive
threshold of hypertensive subjects. Methods: Forty-one participants were
divided into two groups: a normotensive group and hypertensive group. In both
groups, the body mass index, abdominal circumference, systolic and diastolic
blood pressure, heart rate, nociceptive threshold and functional capacity by
six-minute walking test (6MWT) were evaluated. The CRP was composed of aerobic
exercises on a treadmill for 30 min, 3 times a week for 7 weeks. Results:
After the CRP, there was a reduction (p < 0.001) in the mechanical
nociceptive threshold and an increase in the distance walked during the 6MWT (p
< 0.001) in the hypertensive group. No differences were found in the body
mass index between the groups. Conclusion: The results of the present
study suggested that aerobic exercise is an important modality to normalize the
nociceptive threshold and improved the functional capacity of the hypertensive
subjects.
Keywords: cardiac rehabilitation;
hypertension; analgesia.
Resumo
Objetivos: Indivíduos hipertensos apresentam um
limiar nociceptivo aumentado, o que pode contribuir para uma redução da
percepção da dor. Assim, o objetivo deste estudo foi avaliar o efeito de um
programa de reabilitação cardíaca (PRC) sobre limiar nociceptivo aumentados de
indivíduos hipertensos. Métodos: Quarenta e um participantes foram
divididos em dois grupos: grupo normotenso e grupo hipertenso. Nos dois grupos,
foram avaliados a massa corporal, índice de massa corporal (IMC),
circunferência abdominal, pressão arterial sistólica e diastólica, frequência
cardíaca, limiar nociceptivo e a capacidade funcional pelo teste de caminhada
de seis minutos (TC6). O PRC foi composto de exercícios aeróbicos em esteira
com duração de 30 minutos, 3 vezes por semana, durante 7 semanas. Resultados:
Após o PRC, houve uma significativa redução (p < 0,001) no limiar mecânico
nociceptivo e um aumento na distância percorrida durante o TC6 (p < 0,001)
no grupo composto por indivíduos hipertensos. Conclusão: Os resultados
do presente estudo sugeriram que o exercício aeróbico é uma modalidade
importante para normalizar o limiar nociceptivo e melhorar a capacidade
funcional de indivíduos hipertensos.
Palavras-chave: reabilitação cardíaca; hipertensão;
analgesia.
Introduction
According to the World Health
Organization, 330 million people worldwide have hypertension, and this number
could increase to approximately 1.56 billion by the year 2025 [1]. Hypertension
is considered to be a major risk factor for the development of coronary artery
disease (CAD), and therefore, sudden cardiac death [2]. With regard to CAD,
recent studies have shown that a silent ischemic infarction was a common
manifestation in 50% of the subjects [3].
In addition to the risk of
developing CAD, studies have found that hypertensive individuals have a lowered
pain sensitivity [4], which correlates with an increased incidence of silent
ischemic infarctions and heart attacks [5]. Glazier et al. [6] found a
higher pain tolerance in patients who had silent ischemic infarction episodes
when compared to those who had painful ischemic episodes.
Because pain is a one of the body’s
protective mechanisms, it is of paramount importance that its physiology always
functions under normal conditions [7]. Many studies have investigated the
mechanisms responsible for the high pain threshold that occurs at the onset of
hypertension, and several have indicated the role of the baroreflex system in
pain modulation. A reduction or interruption in the afferent activity of the sinoaortic baroreceptors attenuated the increased
nociceptive threshold in several experimental models of hypertension [8,9]. In
addition to the baroreflex system, some endogenous systems may also be involved
in the high nociceptive threshold found in hypertension. Zamir et al.
[10] demonstrated that chronic treatment with naloxone, an antagonist to opioid
receptor, reduced the nociceptive threshold, which was increased in
spontaneously hypertensive rats, suggesting that these peptides participate in
this effect. The renin-angiotensin system has also been suggested, in which
nociceptive threshold normalization was found in spontaneously hypertensive
rats after 3 consecutive days of treatment with captopril, an angiotensin AT1
receptor antagonist [11].
With regard to treatment, regular
physical activity has been used as a nonpharmacological therapeutic strategy
for controlling hypertension [12]. In addition, Galdino
et al. [13] found a reduction in the nociceptive threshold, which was
increased in spontaneously hypertensive rats, after 4 weeks of aerobic
exercise. However, this effect has not been investigated in humans.
Based on the abovementioned
information, the present study aimed to investigate the effects of a cardiac
rehabilitation program (CRP) on the nociceptive threshold and arterial blood
pressure (BP) levels in hypertensive subjects.
Methods
Participants
This study was conducted in
accordance with The Code of Ethics of the World Medical Association
(Declaration of Helsinki) and approved by the research ethics committee of the
university (protocol number: 478.427). All participants signed the informed
consent form and this clinical trial study and was registered at
www.ensaiosclinicos.gov.br (number: RBR-67hvhy, principal investigator: Ana
Clara Desiderio Maldonado, date of registration:
august 04, 2018). In addition, this study complied with the Transparent
Reporting of Evaluations with Nonrandomized Designs statement [14].
From September 2018 to December
2018, 63 participants were assessed and divided into two groups: a hypertensive
group (HG, n = 30) composed of subjects with clinical diagnoses of hypertension
and a normotensive group (NG, n = 11) composed of normotensive subjects. The
use of this group was important for demonstrating how the nociceptive threshold
of normotensive subjects and the effect of exercise on this threshold, besides
comparing hypertensive subjects. Due to the lack of individuals, it was not
possible to have a control group constituted by hypertensives. Thus, the data
obtained before the intervention were used as data referring to the
hypertensive group not exercised.
The hypertension diagnoses were
made by a certified cardiologist according to the criteria established by the
European Society of Hypertension and the European Society of Cardiology [15].
Hypertensive subjects were recruited from cardiology clinics of our University
and the normotensive subjects were recruited through posters and word of mouth.
The enrolled hypertensive subjects had no histories of the following: unstable
angina, thrombophlebitis, recent embolism, acute systemic infection,
third-degree atrioventricular block (without the use of a pacemaker), acute
myocarditis or pericarditis, uncontrolled arrhythmia, mitral stenosis without
adequate treatment, decompensated heart failure, uncontrolled hypertension (SBP
≥ 200 mmHg or DBP ≥ 110 mmHg), ST segment depression > 2 mm,
uncontrolled diabetes mellitus, acute systemic sickness or fever of unknown
origin, and other uncompensated metabolic problems. In addition, for inclusion
in the study, normotensive subjects were required to have BP lower than 140/90
mmHg, whereas hypertensive subjects were required to have BP between 140/90 and
159/99 mmHg. The participants smokers, engaged in regular physical activity,
using pain medication, antidepressants, tranquilizers, or any medicine that
could interfere with the nociceptive threshold, or drugs or alcohol users were
excluded from the study. Moreover, the participants had to present an assiduity
to the CRP > 80%.
Procedures
Before starting the CRP, each of
the patients had an interview with a physiotherapist. The interview questions
concentrated on the hypertension onset time, symptoms, life quality,
pharmacotherapy, physical activity, and work. In addition, anthropometric
measurements of the subjects’ physical characteristics were performed,
including the weight (kg), height (m), and mass by height based on the body
mass index (BMI, kg/m2).
The BP, heart rate (HR), and
nociceptive threshold were also measured, and the functional capacity was
obtained by using the six-minute walk test (6MWT), as described below.
Nociceptive threshold measurement
The mechanical nociceptive
threshold was measured using a pressure algometer (EMG System of Brazil, São
José dos Campos, SP, Brazil). The force was displayed digitally in increments
of 1 kgf, and the cut-off pressure was set at 10 kgf to avoid tissue damage. Pressure was applied to the
skin (right hand extensor region), and when the participant vocally reported
pain, the stimulus was stopped. The value scored on the apparatus was described
as the latency of the nociceptive threshold. The nociceptive threshold was
measured initially and after the CRP in each group of participants. The
threshold measurements were performed in triplicate, and the results were
presented as the mean ± the standard error of the mean (S.E.M).
Evaluation of functional capacity
To evaluate the cardiovascular and
physical conditioning gains, the 6MWT was used. The participants walked along
an enclosed level corridor for 30 meters. They were encouraged and instructed
to walk at their own pace, but to cover as much ground as possible in 6
minutes. They tolerated the 6MWT without any adverse effects. The HR, arterial
BP, rating of perceived exertion (RPE), and arterial oxygen saturation were
measured at rest, continuously every 2 minutes while walking, and after the
test [16].
Cardiac rehabilitation program
The CRP was performed in accordance
with the Guidelines for Rehabilitation in Patients with Cardiovascular Disease
[17]. The participants underwent 20 CRP sessions performed at the physical
therapy clinic of our University. The CRP used in this study was composed of
aerobic exercises on a treadmill (RT250; Movement, Manaus, AM, Brazil). Each
patient started with a 10-minute warm-up at a low velocity, followed by 30
minutes at a velocity that maintained the target HR, and ended with an
appropriate cooling-off period of 10 minutes of slow walking and stretching.
The CRP exercises were conducted 3 times a week for 7 weeks. The patients
exercised at 60% to 70% of their HR calculated according to the Karvonen
formula: [(maximal HR – resting HR × % exercise intensity) + resting HR]. The
HR was measured continuously with a portable HR monitor (RS200; Polar Electro
Inc., Woodbury, NY, USA). The monitor was set to maintain a target HR and to
signal (by a beep) when the HR fell outside of the chosen exercise’s target
range. The BP was monitored periodically during each workout to ensure that it
was within safe limits (SBP ≤ 210 mmHg for the males and ≤ 190 mmHg
for the females and DBP ≤ 110 mmHg for both the males and females) [18].
The workload was adjusted to maintain the target HR and BP within the
prescribed limits throughout the exercise session. The patients were also
subjectively rated using a modified Borg RPE scale [19]. For those participants
who did not reach the target HR, the intensity was determined by a score of 5-7
on the modified RPE scale. The arterial BP, HR, and RPE were measured before,
during (every 10 minutes), and after the exercise program. The patients were
advised to consume water, according to their medical status, before checkout.
The CRP was carried out in the
morning (8 am to 12 pm) in order to minimize the impact of the circadian rhythm
on the cardiovascular variables. The room temperature was maintained at 23°C,
and the relative air humidity was between 40% and 60%. The participants were
acquainted with the experimental protocol, and they were instructed to abstain
from stimulants (coffee, tea, and soft drinks) and alcoholic beverages for 24
hours preceding the CRP. They were advised to have a light meal at least two
hours before the procedure. Additionally, the subjects were asked to avoid
physical activity one day before the procedure.
Statistical analysis
The descriptive data were expressed
as means and standard deviations. The data normality was tested using the
Kolmogorov-Smirnov and Shapiro-Wilk tests, followed by the paired t test for
the intragroup analyses and the independent t test for the intergroup analyses.
The parametric variables were expressed as means and standard deviations, and
the frequencies were expressed by absolute numbers and percentages. For the
sample calculation, we used the results of a previous study [20], considering a
confidence level of 95% and a margin of error of 5%. All statistical analysis
was performed using SPSS v.20.0 (IBM Corp., Armonk, NY, USA).
Results
From a list of approximately 63
individuals, 22 were excluded due to low assiduity (< 80%), resulting in a
total of 41 participants (Fig. 1).
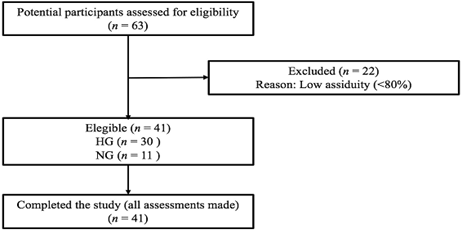
Figure 1 - Flow-chart
Sixty-three participants were
assessed and divided into two groups: a hypertensive group (HG, n = 30)
composed of subjects with clinical diagnoses of hypertension and a normotensive
group (NG, n = 11) composed of normotensive subjects. 22 individuals were
excluded due to low assiduity, resulting in a total of 41 participants.
Regarding baseline clinical
characteristics of the subjects that participated in this study, approximately
53% of the HG was composed of males, with 47% females. In the NG, the male
population was 27% and the female population was 73%. Furthermore, with regard
to age, there were no significant differences between the groups, being the
average age of 61.9 ± 10.4. When evaluating the associated diseases, the HG had
a higher percentage of individuals with associated diseases (8 diabetes
mellitus, 12 sleep disturbances and 12 lung diseases) when compared to the NG
(5 diabetes mellitus, 4 sleep disturbances and 3 lung diseases). In addition,
in the HG, 6 subjects had acute myocardial infarction and 7 were submitted to
cardiac surgery and none of the subjects in the NG had heart disease.
We also evaluated the
anthropometric variables of the groups before and after the CRP. Table I shows
that the baseline values of the body mass (BM) and abdominal circumference (AC)
were higher (p < 0.05 and p < 0.001, respectively) in the HG when
compared to the baseline values of the NG. In addition, the BM and AC values at
the end of the CRP were also higher (p < 0.05 and p < 0.01, respectively)
in the HG.
Table I - Anthropometric variables of
groups before and after CRP
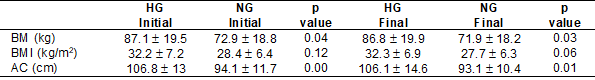
Source: Research data; CRP =
cardiac rehabilitation program; AC = abdominal circumference; BM = body mass; BMI
= body mass index; HG = hypertensive group; NG = normotensive group. Values are
expressed as mean ± standard error (mean ± SEM)
For the intragroup comparisons of
the anthropometric variables, we did not find differences between the baseline
and final values in either group (Table II).
Table II - Intra-group anthropometric
variables of groups before and after CRP
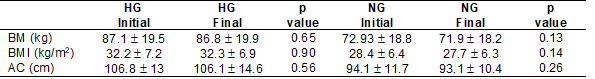
Source: Research data; CRP =
cardiac rehabilitation program; AC = abdominal circumference; BM = body mass;
BMI = body mass index; HG = hypertensive group; NG = normotensive group. Values
are expressed as mean ± standard error (mean ± SEM)
We also analyzed some of the
clinical variables before and after the CRP. In the between-group evaluations,
we verified that the HG presented higher baseline values for the SBP (p <
0.001), DBP (p < 0.05), and mechanical nociceptive threshold (p < 0.01)
when compared to the NG (Table III). Table IV shows also that the 6MWT baseline
values were higher (p < 0.01) in the NG.
After the CRP, the SBP and DBP
values remained higher (p < 0.01 and p < 0.05, respectively) in the HG
when compared to the NG (Table III). For the mechanical nociceptive threshold
and 6MWT values, there were no differences between the groups after the CRP
(Table III).
Table III - Clinical variables,
nociceptive threshold and physical performance of groups before and after CRP
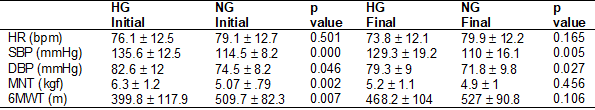
Source: Research data; CRP =
cardiac rehabilitation program; 6MWT = six minutes walking test; SBP = systolic
blood pressure; DBP = diastolic blood pressure; HR = heart rate; HG =
hypertensive group; NG = normotensive group; MNT = mechanical nociceptive
threshold; SBP = systolic blood pressure. Values are expressed as mean ±
standard error (mean ± SEM)
The intragroup analysis of the clinical
variables showed that after the CRP there was a reduction (p < 0.001) in the
mechanical nociceptive threshold and an increase in the distance walked in the
6MWT (p < 0.001) in the HG when compared to the baseline values (Table IV).
In the NG, there were no statistical differences between the clinical variables
before and after the CRP (Table IV).
Table IV - Intra-group clinical
variables, nociceptive threshold and physical performance of groups before and
after CRP

Source: Research data; CRP = cardiac
rehabilitation program; 6MWT = six minutes walking test; DBP = diastolic blood
pressure; HR = heart rate; HG = hypertensive group; NG = normotensive group; MN
= mechanical nociceptive threshold; SBP = systolic blood pressure. Values are
expressed as mean ± standard error (mean ± SEM)
Discussion
The present study determined that
the CRP increased the physical conditioning and normalized the mechanical
nociceptive threshold that was increased in the hypertensive subjects.
In the analysis of the BMI, the HG
presented class 1 obesity and a high CAD risk, whereas the NG was classified as
overweight with a lower cardiovascular risk [21]. The BM, AC, and BMI are not
only indicators of obesity, they are among the factors associated with hypertension.
Moreover, these variables are increased in hypertensive subjects, and they are
risk factors for CAD [22,23]. Thus, corroborated by the evidence previously
cited, we suggest that the HG in the present study had a greater cardiovascular
disease (CVD) risk than the NG.
The BP level evaluation showed that
the SBP and DBP levels were higher in the HG when compared to the NG before and
after the CRP. In accordance with the new high BP clinical guidelines [24], we
suggest that the participants in the HG had stage 1 hypertension. Previous
studies have demonstrated graded associations between higher SBP and DBP levels
and an increased CVD risk [25,26]. Thus, nonpharmacological therapies, such as
exercise, have been used to treat and control this condition.
However, when we investigated the
effects of the CRP on these values, we did not find any statistical differences
in either of the groups. Several factors may have contributed to these results.
First, we believe that the reduced sample size may have interfered with the
results, because there was a tendency toward a reduction in these levels, but
no statistical significance. Another factor could be the aerobic training
duration. In our study, the CRP lasted 7 weeks, but in most of the other studies,
the training lasted 10 weeks [27]. However, our results in the 6MWT
demonstrated that the physical conditioning of the HG improved after the CRP.
Although there was no statistically
significant difference in the BP values after the CRP, there was a reduction in
the SBP (from 135.67 ± 12.51 to 129.33 ± 19.29 in the HG and from 114.54 ± 8.20
to 110.0 ± 16.12 in the NG) and the DBP (from 82.67 ± 12.01 to 79.33 ± 9.07 in
the HG and from 74.54 ± 8.20 to 71.82 ± 9.82 in the NG). Randomized clinical
trials have documented that lowering the BP is associated with reductions in
the CVD and all-cause mortality risks [28,29]. In addition, studies have also
shown a strong, independent, and log-linear association between the normal SBP
and DBP levels and mortality from CVD and all causes with no evidence of a
threshold down to at least 115 mmHg and 75 mmHg, respectively [30]. Moreover,
when following the new high BP clinical guidelines, the BP category in the HG
changed from stage 1 hypertension (SBP from 130-139 mmHg or DPB from 80-89
mmHg) to elevated (less than 120/80 mmHg) [24]. Thus, despite the fact that the
reductions in the BP values after the CRP were not statistically significant,
the program may have helped reduce the BP in the hypertensive subjects, which
reinforces the importance of physical of exercise in this population.
After evaluating the physical
conditioning of the participants in the present study by using the 6MWT, the
results only demonstrated an increase in the distance walked after the CRP in
the HG. However, the baseline values demonstrated that the NG already had good
physical conditioning. The 6MWT is commonly used to assess the fitness level of
healthy adults and older adults with disabilities and pathologies, such as
strokes, chronic obstructive pulmonary disease, pulmonary arterial
hypertension, and heart failure [31,32,33].
The results of the present study
also showed that the hypertensive subjects presented an increase in the
baseline nociceptive threshold in relation to the normotensive subjects. This
finding was first reported by Zamir and Shuber [34]
who measured the pain perception by using electrical stimulation applied to the
tooth pulp of 21 hypertensive and 34 normotensive subjects.
An early explanation for the increased
nociceptive threshold of the hypertensive subjects could be that BP elevations
may activate the baroreceptor pathways and induce a general inhibition of
central nervous system processes and the pain via the activation of the
descending control of pain [35]. One study found that bilateral sinoaortic denervation in spontaneously hypertensive rats
partially reversed the attenuated response of the dorsal horn neurons, which is
consistent with the view of a descending inhibitory baroreflex influence on the
spinal nociceptive transmission in this form of experimental hypertension [36].
The involvement of endogenous
analgesic substances that may be released by the descending control of the pain
pathway are also associated with the increase in the nociceptive threshold
found in hypertensive rats. A study showed an association between an increased
nociceptive threshold and an increase in the β endorphin levels in 20
hypertensive subjects [37]. In addition to the opioid system, one study
demonstrated the participation of angiotensin II in the increased nociceptive
threshold in hypertensive patients [11]. Thus, one possible mechanism to
explain the normalization of the nociceptive threshold in the hypertensive
subjects after chronic physical training could be the reduction of the elevated
levels of the endogenous analgesic substances.
With regard to the occurrence of
silent myocardial ischemia (SMI), which may be associated with the increase in
the nociceptive threshold found in hypertensive individuals, Glazier et al.
[6] found a greater tolerance to pain in those patients with SMI episodes when
compared to those with episodes of painful ischemia. In parallel, Nalbantgil et al. [38] reported that the SMI
prevalence was significantly higher in hypertensive individuals. This occurred
in 26.2% of the hypertensive subjects, 18.8% of the individuals with white coat
hypertension, and 6.4% of the normotensive subjects in their study.
Study limitations
One of the study limitations was
the small sample size of normotensive patients to the cardiac rehabilitation
program due to low adherence, as well as the low assiduity. Although studies
have demonstrated that hypertensive subjects presented hypolgesia
and this finding is associated with SMI, the present study did not have access
to the angina threshold of each individual, evaluated by an ergometric test and
compared this threshold after aerobic training. However, we have shown that
aerobic training reduced the nociceptive threshold increased in hypertensive
individuals and this effect may be related to a reduction of the angina
threshold.
Conclusion
The results of the present study
showed a reduction in the nociceptive threshold of the hypertensive subjects
after aerobic training, suggesting that aerobic exercise can be a strategy of
prevention of SMI. In addition, CRP was efficient in control the arterial BP
and improve the physical conditioning in hypertensive subjects. Further
investigations are needed to elucidate the specific mechanisms that contribute
increased nociceptive threshold in hypertensive subjects and the control of
this alteration by exercise.
Conflicts of interest
The authors report no conflicts of
interest.
Funding source
This study was supported by
National Council for Scientific and Technological Development (CNPq), Foundation for Research Support of the State of
Minas Gerais (FAPEMIG) and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES)
[Grant 001].
Author' contributions
Conception and research design:
Maldonado ACD, Galdino G; Data
collection: Maldonado ACD; Prado JP; Data analysis and interpretation:
Maldonado ACD, Aquino TN, Galdino G; Statistical
analysis: Aquino TN, Vidigal FC; Writing of the
manuscript: Maldonado ACD; Galdino G; Borges JBC;
Critical revision of the manuscript for important intellectual content: Galdino G, Borges JBC, Prado JP
References
- Whitworth JA. World Health Organization, International
Society of Hypertension Writing Group. 2003 World Health Organization
(WHO)/International Society of Hypertension (ISH) statement on management of
hypertension. J Hypertens 2003;21:1983-92. doi: 10.1097/00004872-200311000-00002 [Crossref]
- Jensen JS, Feldt-Rasmussen B, Strandgaard S, Schroll M, Borch-Johnsen K. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension 2000;35:898-903. doi: 10.1161/01.hyp.35.4.898 [Crossref]
- Chen CC, Chong CF, Kuo CD, Wang TL. Silent myocardial ischemia in coronary artery disease patients under aspirin therapy presenting with upper gastrointestinal hemorrhage. J Gastroenterol Hepatol 2007;22:13-17. doi: 10.1111/j.1440-1746.2006.04277.x [Crossref]
- Ghione S, Rosa C, Mezzasalma L, Panattoni E. Arterial hypertension is associated with hypalgesia in humans. Hypertension 1988;12:491-7. doi: 10.1161/01.hyp.12.5.491 [Crossref]
- Modolo R, Faria AP, Paganelli MO, Sabbatini AR, Barbaro NR, Nascimento BB. Predictors of silent myocardial ischemia in resistant hypertensive patients. Am J Hypertens 2015;28:200-7. doi: 10.1093/ajh/hpu140 [Crossref]
- Glazier JJ, Chierchia S, Brown MJ, Maseri A. Importance of generalized defective perception of painful stimuli as a cause of silent myocardial ischemia in chronic stable angina pectoris. Am J Cardiol 1986;58:667-672. doi: 10.1016/0002-9149(86)90335-8 [Crossref]
- Bonica JJ, Loeser JD. History of pain concepts and therapies. In: Loeser JD, Butler SH, Chapman CR, et al. Bonica's Management of Pain. Philadelphia, PA: Lippincott;
2001. p.3-16.
- Randich A, Maixner W. Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev 1984;8:343-367. doi: 10.1016/0149-7634(84)90057-5 [Crossref]
- Randich A, Maixner W. The role of sinoaortic and cardiopulmonary baroreceptor reflex arcs in nociception and stress-induced analgesia. Ann N Y Acad Sci 1986;467:385-401. doi: 10.1111/j.1749-6632.1986.tb14642.x [Crossref]
- Zamir N, Simanto R, Segal M. Pain sensitivity and opioid activity in genetically and experimentally hypertensive rats. Brain Res 1980;184:299-310. doi: 10.1016/0006-8993(80)90800-8 [Crossref]
- Irvine RJ, White JM, Head RJ. The renin angiotensin system and nociception in spontaneously hypertensive rats. Life Sci 1995;56:1073-78. doi: 10.1016/0024-3205(95)00043-6 [Crossref]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 2004;36:533-553. doi: 10.1249/01.mss.0000115224.88514.3a [Crossref]
- Galdino GS, Lopes AM, Franca VM, Duarte IDG, Andrea C Perez AC. Evaluation of exercise and potassium chloride supplementation on blood pressure and nociceptive threshold in hypertensive rats. Appl Physiol Nutr Metab 2010;35:184-7. doi: 10.1139/h09-138 [Crossref]
- Des Jarlais DC, Lyles C, Crepaz N. The TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND Statement. Am J Public Health 2004;94:361-6. doi: 10.2105/ajph.94.3.361 [Crossref]
- Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M. Task Force Members. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281-1357. doi: 10.1097/01.hjh.0000431740.32696.cc [Crossref]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111-117. doi: 10.1164/ajrccm.166.1.at1102 [Crossref]
- JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014;78:2022-93. doi: 10.1253/circj.cj-66-0094 [Crossref]
- Lauer MS, Levy D, Anderson KM, Plehn JF. Is there a relationship between exercise systolic blood pressure response and left ventricular mass? The Framingham Heart Study. Ann Intern Med 1992;116:203-10. doi: 10.7326/0003-4819-116-3-203 [Crossref]
- Borg G. Borg's perceived exertion and pain scales. 1st
Ed. Champaign, IL: Human Kinetics; 1998.
- Jurio-Iriarte B, Maldonado-Martín S. Effects of different exercise training programs on cardiorespiratory fitness in overweight/obese adults with hypertension: a pilot study. Health Promot Pract 2018;1:1524839918774310. doi: 10.1177/1524839918774310 [Crossref]
- World Health Organization. Defining the problem of
overweight and obesity. In: World Health Organization, ed. Obesity: preventing
and managing the global epidemic: report of a Who Consultation. Geneva, GE: WHO
Technical Report Series; 2000:241-3.
- Harris MM, Stevens J, Thomas N, Schreiner P, Folsom AR. Associations of fat distribution and obesity with hypertension in a bi-ethnic population: The ARIC Study l. Obes Res 2000;8:516-24. doi: 10.1038/oby.2000.64 [Crossref]
- Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004;79:379-84. doi: 10.1093/ajcn/79.3.379 [Crossref]
- Whelton PK, Carey RM, Aronow WS, Casey Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:1269-1324. doi: 10.1161/hyp.0000000000000066 [Crossref]
- Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med 1993;153:598-615. doi: 10.1001/archinte.153.5.598 [Crossref]
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Spiros Denaxas S. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899-1911. doi: 10.1016/s0140-6736(14)60685-1 [Crossref]
- Wen H, Wang L. Reducing effect of aerobic exercise on blood pressure of essential hypertensive patients: A meta-analysis. Medicine 2017;96:6150. doi: 10.1097/md.0000000000006150 [Crossref]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al.
Joint National Committee on Prevention, Detection, Evaluation, and Treatment of
High Blood Pressure. National Heart, Lung, and Blood Institute; National High
Blood Pressure Education Program Coordinating Committee. Seventh report of the
Joint National Committee on Prevention, Detection, Evaluation, and Treatment of
High Blood Pressure. Hypertension 2003;289:2560-72.
- Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively designed overviews of randomized trials. Lancet 2003;362:1527-35. doi: 10.1016/s0140-6736(03)14739-3 [Crossref]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. doi: 10.1016/s0140-6736(02)11911-8 [Crossref]
- Demers C, McKelvie RS, Negassa A, Yusuf S. RESOLVD Pilot Study Investigators. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J 2001;142:698-703. doi: 10.1067/mhj.2001.118468 [Crossref]
- Chen H, Liang BM, Tang YJ, Xu ZB, Wang K, Yi Q, et al.
Relationship between 6-minute walk test and pulmonary function test in stable
chronic obstructive pulmonary disease with different severities. Chin Med J
[Internet]. 2012 [cited 2022 May 16];125:3053-8.
Available from: https://pubmed.ncbi.nlm.nih.gov/22932179/
- Mehta S, Pereira S, Janzen S, Mays R, Viana R, Lobo L, et al. Cardiovascular conditioning for comfortable gait speed and total distance walked during the chronic stage of stroke: a meta-analysis. Top Stroke Rehabil 2012;19:463-70. doi: 10.1310/tsr1906-463 [Crossref]
- Zamir N, Shuber E. Altered pain perception in hypertensive humans. Brain Res 1980;201:471-4. doi: 10.1016/0006-8993(80)91055-0 [Crossref]
- Dworkin BR, Elbert T, Rau H, Birbaumer N, Pauli P, Droste C, et al. Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proc Nati Acad Sci U S A 1994;91:6329-33. doi: 10.1073/pnas.91.14.6329 [Crossref]
- Randich A, Robertson JD. Spinal nociceptive transmission in the spontaneously hypertensive and Wistar-Kyoto normotensive rat. Pain 1994;58:169-83. doi: 10.1016/0304-3959(94)90197-x [Crossref]
- Vignocchi G, Murri L, Rossi B, Rosa C, Ghione S. Correlation between
pain thresholds and polysinaptic components of the
blink reflex in essential arterial hypertension. Funct
Neurol [Internet] 1989 [cited 2022 May 20];4:59-61.
Available from: https://pubmed.ncbi.nlm.nih.gov/2737495/
- Nalbantgil I, Onder R, Nalbantgil S, Yilmaz H, Boydak B. The prevalence of silent myocardial ischaemia in patients with white-coat hypertension. J Hum Hypertens 1998;12:337-341. doi: 10.1038/sj.jhh.1000607 [Crossref]
