Fisioter Bras 2022;23(6):841-52
ORIGINAL ARTICLE
Urinary incontinence and sexual dysfunction in type 1 diabetic pregnant
women: preliminary results
Incontinência urinária
e disfunção sexual em gestantes diabéticas tipo 1: resultados preliminares
Patricia Andrade Batista*, Cláudia de
Oliveira**, Kérolin L. Carvalho Kaneto***,
Rafaela Alkmin da Costa*, Fabio Roberto Cabar*,
Clarice Tanaka***, Rossana Pulcineli
Vieira Francisco*
*Departamento de
Ginecologia e Obstetrícia, Faculdade de Medicina, Universidade de São Paulo
(USP), São Paulo, SP, Brazil, **Departamento de
Fisioterapia, Universidade Santa Cecília (UNISANTA), Santos, SP, Brazil, ***Departamento de Fisioterapia, Fonoaudiologia e
Terapia Ocupacional, Faculdade de Medicina, Universidade de São Paulo (USP),
São Paulo, SP, Brazil
Received: February 22,
2022; Accepted: November
23, 2022.
Correspondence: Patricia
Andrade Batista, Avenida Antônio Frederico Ozanan,
9300, apto 71 bloco 1, Jundiaí SP, Brazil
Patricia Andrade Batista:
pab.fisio@gmail.com
Claudia de
Oliveira: claufisio2005@yahoo.com.br
Kerolin L. Carvalho Kaneto: sm.kerolinkaneto@gmail.com
Rafaela
Alkmin da Costa: rafaela.alkmin@hc.fm.usp.br
Fabio
Roberto Cabar: fabio.cabar@hc.fm.usp.br
Clarice
Tanaka: cltanaka@usp,br
Rossana Pulcineli
Vieira Francisco: rossana.francisco@hc.fm.usp.br
Abstract
Introduction: Pregnancy and diabetes mellitus can promote various
musculoskeletal disorders, predisposing individuals to urinary and sexual
dysfunction. Objective: To evaluate the frequency of urinary
incontinence and sexual dysfunction in pregnant women with type 1 diabetes. Methods:
A cross-sectional, observational, analytical study was conducted. Thirteen
pregnant women with type 1 diabetes were assessed from April 2017 to February
2018 using the International Consultation on Incontinence Questionnaire-Short
Form (ICIQ-SF) and the Female Sexual Function Index (FSFI). The statistical
program SPSS version 20.1 was used for data analysis to determine the mean and
standard deviation, central tendency and dispersion, and absolute (n) and
relative (%) frequencies. The Pearson linear correlation test was used to
evaluate the correlation between the ICIQ-SF and the FSFI. Results: The
frequency of urinary incontinence was 38.5%, which produced a slight impact on
the quality of life of pregnant women. The FSFI general score was 20.49,
identifying the presence of sexual dysfunction (FSFI ≤ 26). In the group
of pregnant women, 92.3% (n = 12) presented sexual dysfunction, and the
satisfaction and arousal domains showed a higher frequency of problems. The
ICIQ-SF and FSFI showed a moderate but not significant correlation (r = 0.534,
p = 0.60). The data exhibited a normal distribution according to Levene’s test. Conclusion: The frequency of urinary
incontinence in pregnant women with type 1 diabetes was low, but sexual
dysfunction was present, and the satisfaction and arousal domains showed the
highest frequency of problems.
Keywords: sexuality; urinary incontinence; diabetes mellitus.
Resumo
Introdução: A gestação e o diabetes mellitus
promovem diversas alterações musculoesqueléticas, predispondo disfunções
miccionais e sexuais. Objetivo: Avaliar a frequência de incontinência
urinária e disfunção sexual em gestantes diabéticas tipo 1. Métodos:
Estudo analítico, observacional do tipo transversal. Foram avaliadas 13
gestantes com diabetes do tipo 1 no período de abril 2017 a fevereiro 2018, por
meio dos Questionários International Consultation on Incontinence Questionnaire -
Short Form (ICIQ-SF) e o Female
Sexual Function Index (FSFI). Foi utilizado o programa
estatístico SPSS versão 20.1 para análise de dados, avaliando a média e desvio
padrão (DP), tendência central e dispersão, frequência absoluta (n) e relativa
(%). Para correlação entre o ICIQ e o FSFI o teste de correlação linear de
Pearson. Resultados: Frequência de incontinência urinária foi 38,5%, o
que demonstrou impacto leve na qualidade de vida das gestantes. O escore geral
do FSFI foi de 20,49, identificando a presença de disfunção sexual (FSFI ≤
26). No grupo de gestantes, 92,3% (n = 12) apresentaram disfunção sexual, os
domínios satisfação e excitação com maiores prevalências. Correlacionando o
ICIQ-SF com FSFI, houve correlação moderada, mas não significativa (r = 0,534;
p = 0,60). As amostras apresentaram distribuição normal de acordo com o teste
de Levene. Conclusão: A frequência de incontinência urinária em
gestantes diabéticas do tipo 1 foi baixa, mas houve presença de disfunção
sexual, os domínios satisfação e excitação foram os mais frequentes.
Palavras-chave: sexualidade; incontinência urinária;
diabetes mellitus.
Introduction
Diabetes mellitus type 1 (DM1) can occur at any age
but usually manifests in individuals younger than 30 years, mainly during
school age and adolescence [1]. It is a metabolic disease determined by defects
in the secretion or action of insulin or both that cause chronic hyperglycemia
with long-term damage, leading to dysfunction and failure in different organs
[2].
The incidence of diabetes mellitus (DM) during
pregnancy parallels its prevalence in women of reproductive age. It is
estimated that 2-5% of all pregnant women are affected by DM and its maternal
and fetal complications [3]. In addition to complications related to pregnancy,
these women may also present an exacerbation of complications related to
diabetes, such as retinopathies, neuropathies and chronic hypertension [2]. DM
affects multiple organ systems including the urinary system in approximately
52% of diabetic patients and those with only hyperglycemia. Urinary
incontinence (UI) is common among women with DM1, and risk factors may include
advanced age, weight gain and previous urinary tract infection [4].
According to the International Continence Society, UI
consists of involuntary loss of urine, which causes a public health problem
that can negatively interfere with the quality of life of women [5]. Many risk
factors are involved in the development of UI, but the association with DM is
currently of great interest. The occurrence of UI during pregnancy is also high
[6].
During pregnancy, the increase in maternal body weight
and the weight of the gravid uterus increases the pressure on the pelvic floor
muscles (PFMs). The increase in body mass index (BMI) during gestation,
multiparity, vaginal delivery, prolonged time of the second period of labor and
episiotomy are factors that decrease the strength of the PFMs and can cause UI
and sexual dysfunctions (SDs) [5,7].
The presence of urinary symptoms as well as changes
during the gestational period can modify sexual function of women, triggering
some types of SDs [5].
Sexual health is also an important aspect of quality
of life in women and is defined by the World Health Organization as a state of
physical, emotional, mental and social well-being [8]. Women experience
sexuality in different ways during various periods of life, especially during
pregnancy, when sexual function and quality of life may be compromised due to
alterations in their body image and lack of PFM recruitment [5,9]. In Brazil,
two studies showed that the sexual function of healthy Brazilian pregnant women
became more compromised as the time of delivery approached [4,10].
Both gestation and DM promote several musculoskeletal
changes that lead to changes in PFM recruitment, predisposing women to voiding
and SD. Thus, it is important to investigate the relationship between DM1 and
gestation to prevent impairment of functionality and better target clinical
interventions during the postpartum period.
The objective of this study is to determine the
frequency of UI and SD in pregnant women with DM1.
Methods
This is an analytical, observational and
cross-sectional study. It was conducted in the Physical Therapy Clinic of the
Obstetric Clinic of the Clinics Hospital School of Medicine of the Universidade de São Paulo (FMUSP) from April 2017 to
February 2018 and evaluated 13 pregnant women with DM1 through questionnaires.
This study was approved by the ethics and human
research committee of the Universidade de São Paulo
under number CAAE1.552.712 (approval date 05/20/2016). The participants were
informed about the study and signed the informed consent form, in compliance
with Resolution 466/2012 of the National Health Council.
Inclusion criteria
Pregnant women diagnosed with DM1; gestational age
between 20 and 24 weeks; (period in which there is a physiological reduction of
the PFM strength and a gradual stabilization of relaxing concentrations) [11];
single fetus gestation; age between 18 and 37 years old at the time of
admission to the study, considered the ideal age for the reproductive period
[12]; and all who read, agreed and signed the informed consent form.
Exclusion criteria
Important orthopedic changes (scoliosis, lower limb
discrepancy) and neurological antecedents that caused cognitive impairment or
motor deficits of the lower limbs.
We used convenience sampling to recruit participants
who were patients in prenatal follow-up at the Endocrinopathy Outpatient
Clinic. After they were included in this study, they were referred to an interview
with the researching physiotherapist on the day of the routine consultation.
Those who were not available to be interviewed at that time were scheduled for
the day of the next prenatal consultation.
Evaluation tools
The pregnant women were evaluated using
questionnaires.
The identification
questionnaire gathered information including sociodemographic data (age,
gestational age, pre-gestational weight, current weight, height, education,
marital status and profession) and health conditions (gestational history). The
International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF),
which was translated and validated into Portuguese by Tamanini
et al. [13], was used for UI evaluation. In a simple, brief and
self-administered manner, the ICIQ-SF evaluates the frequency and amount of
urine loss, the conditions of urine loss, the interference of this condition in
activities of daily living, and the impact of UI on quality of life. The
ICIQ-SF was chosen because of its suitability for Brazilian society. The
questionnaire defines the results as follows: 0, no impact; 1 to 3, light
impact; 4 to 6, a moderate effect; 7-9, severe impact and above 10, very severe
impact [10].
The Female Sexual Function Index (FSFI) questionnaire
which was translated and validated for Brazilian Portuguese pregnant women by Leite et al. [14] and aims to evaluate sexual
function was used. It can be self-administered by women who have had sexual
activity in the last four weeks. It consists of 19 questions that assess the
female sexual response in six domains: sexual desire, sexual arousal, vaginal
lubrication, orgasm, sexual satisfaction and pain. The response options are
scored between 0 and 5, in an increasing manner, except for the questions about
pain, in which the score is defined inversely. The total score is the sum of
scores for each domain multiplied by the factor corresponding to each domain.
The minimum score is 2, and the maximum score is 36 [11]. A total score less
than or equal to 26 was considered a risk for SD.
Statistical analysis
The statistical analysis was performed using SPSS
version 20.1 for Windows statistical software. Continuous variables are
presented as means and standard deviations (sd) or
are expressed as the central tendency and dispersion. Nominal categorical variables
are presented as absolute (n) and relative (%) frequencies. Pearson's linear
correlation test was used to examine the correlation between the ICIQ-SF and
the FSFI. Levene’s test was used to assess the
normality of the data. A value of p = 0.05 was considered statistically
significant.
Results
The sample analysis showed that the mean age of the 13
participants was 29.08 ± 4.6 years old. The average gestational age for the
participants at study enrollment was the 21 ± 1.5st gestational week.
Table I shows the gestational history data; 38.46% (n
= 5) of the participants were primiparas, and 61.53% (n = 8) were multiparas.
These two groups had the same rate of normal deliveries 38.5% (n = 5) and
cesarean deliveries 38.5% (n = 5), and abortion was reported in 23% (n = 3) of
the participants. The majority of pregnant women had a stable partner (92.3%, n
= 12); regarding education levels, 61.6% (8) of the participants had completed
secondary school and had higher education. Regarding the BMI, the majority of
the participants presented adequate weight (46.20%) (6), 38.50% (5) were
underweight, 7.70% (1) were overweight and 7.70% (1) were obese.
The overall FSFI score was 20.49 ± 3.38, which
identified the presence of SD (FSFI ≤ 26) in 92.3% (n = 12) of the
pregnant women.
Analysis of the results presented in Table II showed
that the domains with higher prevalence of SD (domain score ≤ 3.6) were
related to satisfaction (84.61%, n = 11) and arousal (76.92%, n = 10), as they
presented lower means in relation to the other evaluated domains.
The mean ICIQ-SF score was 3.85 ± 5.843, demonstrating
a slight impact of UI on participants' quality of life.
Table III describes question 6 of the ICIQ-SF
questionnaire regarding the frequency of urinary loss. The responses "I
lose urine when coughing or sneezing" and "I lose urine without an
obvious reason" were stated by 23.1% (n = 3) and 15.4% (n = 2) of
participants, respectively.
The ICIQ-SF and FSFI showed a moderate but not
significant correlation (r = 0.534, p = 0.60).
Table I - Analysis of sociodemographic data of pregnant
women with type 1 diabetes (n = 13)
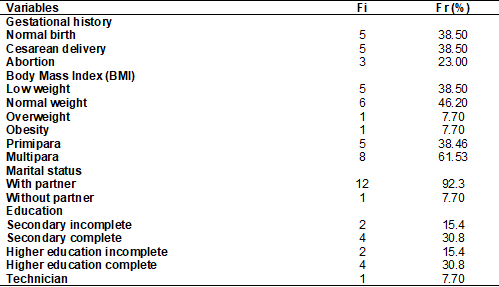
Fi = absolute frequency; Fr = relative frequency; % =
percentage
Table II - Total score and FSFI domains in pregnant women,
presented as the mean and standard deviation (n = 13)
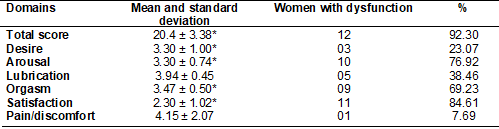
* = domains that showed results below the cutoff
point, predicting some type of sexual dysfunction
Table III - Analysis of question 6 (When do you lose urine?)
of the ICIQ-SF in pregnant women with type 1 diabetes (n = 13)

Fi = absolute frequency; Fr = relative frequency; % =
percentage
Discussion
Although several studies have analyzed UI and sexual
function during pregnancy, we did not find studies correlating UI with SD in
pregnant women with DM1. Based on the results of the present study, the
frequency of UI is low in pregnant women with DM1, and this caused a slight
impact on their quality of life. However, SD was present in the majority of
participants studied. The ICIQ-SF and FSFI values showed a moderate but not
significant correlation (r = 0.534, p = 0.60).
According to a study by Marini et al. [4], UI
was reported by 65% of women with DM1. Of these, 40% were very uncomfortable
with their UI, and 9% believed that this negatively affected their daily
activities; UI was characterized as more severe in women with DM. The present
study did not observe a severe effect of UI in pregnant women with DM1, but it
was associated with a negative impact on the participants' quality of life.
Moccellin et
al. [16] studied 40 pregnant women aged between 18 and 40 years and found
that pregnant women with UI reported that it had a negative impact on their
quality of life compared to women without urinary loss. In the present study,
we also observed a negative impact of UI on the quality of life of pregnant
women. This finding corroborated the findings of a study by Lopes and Higa [17], who analyzed 14 studies and demonstrated the
effects of UI on quality of life. The results revealed that patients suffer
social consequences, negative feelings and/or shame in 8% to 74% of cases, with
moderate to severe impact on quality of life in 10% to 22% of patients. In
addition, UI interfered in marital and sexual life in 7.5% to 33% of subjects.
Mathias et al. [5] conducted a survey to
determine the frequency of UI and SD in women during the third trimester and in
the sixth month postpartum. Among 54 participants, 37% had UI during the third
gestational trimester, and 14.8% had UI six months postpartum. Regarding the
frequency of SD, 42.6% and 32.6% of participants reported SD during pregnancy
and after delivery, respectively. These values are consistent with the present
study, which showed UI in 38.5% and SD in 92.3% of participants.
In this study, we observed a correlation between the
ICIQ-SF and FSFI; that is, pregnant women with DM1 who presented UI showed a
moderate chance of presenting a decrease in sexual quality of life, in some
cases even giving up having intercourse. Those who still had intercourse had
less satisfaction, perhaps due to the possibility of UI occurring during
intercourse. UI leads to embarrassment and causes women to avoid sexual
intercourse because of shame, and this may be one of the reasons why the
arousal domain was diminished. We observed that not all women lose urine during
intercourse, but the presence of UI causes women to prefer to avoid contact
with their partner.
A study by Frigo et al.
[18] found that UI can occur due to functional changes that may have an organic
or psychosocial cause, possibly leading to development of SD, i.e., a total or
partial blockage of the normal sexual response. Satisfaction in this aspect may
be affected not only by the presence of physiological changes but also by the
emotional consequences, loss of self-esteem, shame and feelings of social
inadequacy. This corroborates the analysis of the current study, where a higher
frequency of SD was observed in the domains arousal
and satisfaction in pregnant women with DM1.
According to Rudge and Girão [6], DM is also associated with bladder dysfunction,
including sensory abnormalities that result in impairment of bladder
sensitivity, increased compliance and residual volume and the presence of UI
and SD.
In contrast to the results
of other studies, Mathias et al. [5] found that the relationship between
BMI and parity aggravates or contributes to the development of UI. An
association exists between weight gain as well as the number of previous
deliveries and the prevalence of UI during the gestational period. The present
study did not find any association between BMI and UI or SD.
Marini et al. [4] reported that during
pregnancy, dysfunctions similar to DM occur, in which sexual function changes
during gestation, leading to an increase in SD symptoms, such as decreased
desire and dyspareunia. As the most prevalent SD types, hypoactive desire and
sexual arousal were the most frequent symptoms that persisted during the
postpartum period.
Literature demonstrates and our study finds evidence
that SD can be caused by the presence of DM1 which, due to the extended period
of high glycemia, can damage the nerves and blood vessels of the body leading
to functional deficiencies in various organs, such as the sexual organs. In
addition, difficulties in managing DM can also cause stress and depression,
which can both decrease sexual desire. The use of antidepressant medications
can decrease libido by interfering with sexual function. This analysis
corroborates the findings of a study by Abdo [19] that reported that desire is
regulated by important issues to women in addition to physiological changes,
such as body self-image, mental health and marital relationship, and that women
with genital arousal disorder may present estrogen deficiency or rarer
conditions such as connective tissue disorder.
Lima et al. [20] evaluated the prevalence of SD
before and during pregnancy in primiparas. Among 778 primiparas, 23.9% had SD
before pregnancy, and 67.7% had SD during pregnancy. Before gestation, the rate
of participants reporting a lack of sexual desire was 20.2%, and during
pregnancy, the rate was 51%. Additionally, 29.1% of participants reported a
decrease in vaginal lubrication during gestation; 1.2% reported dyspareunia
before gestation; and 14.4% reported dyspareunia during gestation; 3.3% had
sexual dissatisfaction before pregnancy, and 10.8%, had sexual dissatisfaction
during gestation. These results corroborate the present results regarding the
lack of desire and satisfaction during pregnancy, which were more prevalent
than problems in other domains.
Leite et
al. [14] reported that the importance of sexual health for quality of life
has been increasingly recognized in recent years. SD can have a greater impact
on women's quality of life because diminishing sexual function can have
damaging effects on self-esteem and interpersonal relationships, with frequent
emotional burnout.
The information in the present study is fundamental
when filling the gaps in the lack of studies which evaluate the frequency of UI
and SD in Brazilian pregnant women with DM1, since in other populations such as
pregnant women with DM2 and with low-risk pregnancy reported [21,22].
This study was the first to assess the frequency of
pain and UI in Brazilian pregnant women with type 1 diabetes. Most studies in
pregnant women with diabetes refer to gestational diabetes. It is also worth
mentioning that these are preliminary results and until the end of the data
collection we may have more information to ascertain the data with greater
clarity.
As in other studies, our research had limitations such
as: the sample size, as it is a condition of low prevalence during pregnancy,
around 1 to 2%; the absence of follow-up of these patients, which would make it
possible to verify the impact of these changes on more advanced gestational
ages; the fact that the frequency of these symptoms in the pre-pregnancy period
is not evaluated, which could justify the increased frequency of this
complaint; and the fact that we did not find studies with a similar research
population to compare our findings.
In view of the above, it is expected that the
frequency of UI and SD found in this study can demonstrate the importance of
early diagnosis in pregnant women with DM1 to avoid future complications, as
well as lead to the early implementation of strategic intervention measures for
this population.
Conclusion
The frequency of urinary incontinence in pregnant
women with type 1 diabetes was low, but sexual dysfunction was present, and the
satisfaction and arousal domains showed the highest frequency of problems.
Potential conflict of interest
No potential conflicts of interest relevant to this
article have been reported.
Financing source
There were no external funding sources for this study.
Authors' contribution
Research conception and design: Batista PA, Oliveira C, Francisco RPV; Obtaining data: Batista
PA, Kaneto KC; Data analysis and interpretation:
Batista PA, Oliveira C, Francisco RPV; Statistical analysis: Batista PA,
Oliveira C; Writing of the manuscript: Batista PA; Critical review of
the manuscript for important intellectual content: Oliveira C, Costa RA, Cabar FR, Tanaka C, Francisco RPV
This work
was performed at the Ambulatório de
Fisioterapia da Clínica Obstétrica do Hospital das Clínicas da Faculdade de
Medicina da Universidade de São Paulo, FMUSP, Av. Dr. Enéas de Carvalho Aguiar,
255, Cerqueira César, 05403-000 São Paulo, SP, Brazil
References
- Guizzo S, Patrelli
TS, Rossanese M, Noventa M, Berretta
R, Di Gangi S et al. An update on
diabetic women obstetrical outcomes linked to preconception and pregnancy
glycemic profile: a systematic literature review. The Scientific
World Journal 2013. https://doi.org/10.1155/2013/254901 [Crossref]
- Silveira VMF, Menezes AMB, Post CLA, Machado EC. Uma amostra de pacientes com diabetes tipo 1 no Sul do Brasil. Arq Bras Endocrinol Metab 2001;45(5). https://doi.org/10.1590/S0004-27302001000500005 [Crossref]
- Corrêa FHS, Gomes MB. Acompanhamento ambulatorial de gestantes com diabetes mellitus no Hospital Universitário Pedro Ernesto – UERJ. Arq Bras Endocrinol Metab 2004;48(4). https://doi.org/10.1590/S0004-27302001000500005 [Crossref]
- Marini G, Piculo F, Barbosa AMP, Damasceno DC, Calderon IMP, Matheus SMM et al. Diabetes na gestação e incontinência urinária: uma associação pouco reconhecida. Fisioter Bras 2012;13(1). https://doi.org/10.33233/fb.v13i1.466 [Crossref]
- Mathias AERA, Pitangui ACR,
Macedo LF, Dias TG. Incontinência urinária e disfunção sexual no terceiro
trimestre gestacional e seis meses após o parto. Rev Fisioter S Fun Fortaleza [Internet] 2015[cited 2022 Nov
23];4(2):21-31. Available from: https://repositorio.ufc.br/bitstream/riufc/18564/1/2015_art_aeramathias.pdf
- Rudge MVC, Girão MJBC. Diabetes gestacional e incontinência urinária: interação entre a ginecologia e a obstetrícia. Rev Bras Ginecol Obstet 2011;33(5):207-10. https://doi.org/10.1590/S0100-72032011000500001 [Crossref]
- Barbosa AMP, Carvalho LR, Martins AMVC, Calderon IMP, Rudge MVC. Efeito da via de parto sobre a força muscular do assoalho pélvico. Rev Bras Ginecol Obstet 2005;27(11):677-82. https://doi.org/10.1590/S0100-72032005001100008 [Crossref]
- Wallwiener S, Müller M, Doster A, Kuon RJ, Plewniok K, Feller S, et al. Atividade sexual e disfunção sexual de mulheres no período perinatal: estudo longitudinal. Arch Gynecol Obstet 2017;295(4):873-83. https://doi.org/10.1007/s00404-017-4305-0 [Crossref]
- Ferreira DQ, Nakamura UM, Souza E, Corintio Neto M, Ribeiro MC, Santana TGM, et al. Função sexual e qualidade de vida em gestantes de baixo risco. Rev Bras Ginecol Obstet 2012;34(9):409-13. https://doi.org/10.1590/S0100-72032012000900004 [Crossref]
- Barbosa AM, Dias A, Marini G et al. Urinary incontinence and vaginal squeeze pressure two years post-cesarean delivery in primiparous women with previous gestational diabetes mellitus. Clinics (Sao Paulo) 2011;66:1341-6. https://doi.org/10.1590/S1807-59322011000800006 [Crossref]
- Petersen LK, Vogel I, Agger AO, Westergard J, Nils M, Uldbjerg N. Variations in serum relaxin (hRLX-2) concentrations during human pregnancy. Acta Obstet Gynecol Scand 1995;74:251-6. https://doi.org/10.3109/00016349509024444 [Crossref]
- Zugaib M, Francisco RPV. Obstetrícia. 3 ed.
São Paulo: Manole; 2015.
- Tamanini JTN, Dambros
M, D’Ancona CAL, Palma PCR, Netto Jr NR. Validação para o português do “International Consultation on Incontinence Questionnaire Short-Form”
(ICIQ-SF). Rev Saúde Pública [Internet] 2004 [cited 2022 Nov 23];38(3). Available from:
https://scielosp.org/article/rsp/2004.v38n3/438-444
- Leite APL, Moura EA, Campos AAS, Mattar R, Souza E, Camano L. Validação do Índice da Função Sexual Feminina em grávidas brasileiras. Rev Bras Ginecol Obstet [Internet] 2007 [cited 2022 Nov 23];29(8):414-9. https://doi.org/10.1590/S0100-72032007000800003
- Hentschel H, Alberton DL, Capp E, Goldim JR, Passos EP. Validação do Female
Sexual Function Index (FSFI) para o uso da língua
portuguesa. Rev HCPA [Internet] 2007[cited 2022 Nov
23];27(1):10-4. Available from: https://www.lume.ufrgs.br/handle/10183/164528
- Moccellin AS, Rett MT, Driusso P. Incontinência urinária na gestação: implicações na qualidade de vida. Rev Bras Saúde Matern Infant [Internet] 2014 [cited 2022 Nov 23];14(2):147-54. https://doi.org/10.1590/S1519-38292014000200004
- Lopes MHBM, Higa R. Restrições causadas pela incontinência urinária à vida da mulher. Rev Esc Enferm USP [Internet] 2006 [cited 2022 Nov 23];40(1):34-41. Available from: https://doi.org/10.1590/S0080-62342006000100005
- Frigo LF, Bitencourt TF, Pivetta HMF. A influência da incontinência urinária na satisfação sexual e na qualidade de vida em mulheres climatéricas. Rev Epidemiol Control Infect 2014;4(4):233-7. https://doi.org/10.17058/reci.v4i4.4777 [Crossref]
- Abdo CHN. Considerações a
respeito do ciclo de resposta sexual da mulher: uma nova proposta de
entendimento. Diagn Tratamento [Internet]
2010 [cited 2022 Nov 23];15(2):88-90. Available from:
http://files.bvs.br/upload/S/1413-9979/2010/v15n2/a88-90.pdf
- Lima AC, Dotto LMG, Mamede MV. Prevalência de disfunção sexual em primigestas, no Município de Rio Branco, Acre, Brasil. Cad Saúde Pública 2013;29(8):1544-54. https://doi.org/10.1590/0102-311X00164012 [Crossref]
- Oliveira C, Seleme M, Cansi PF, Consentino RFDC, Kumakura FY, Moreira GA, Berghmans B. Urinary incontinence in pregnant women and its relation with socio-demographic variables and quality of life. Rev Assoc Med Bras 2013;59(5):460-6. https://doi.org/10.1016/j.ramb.2013.08.002 [Crossref]
- Brown JS, Vittinghoff E, Lin F, Nyberg LM, Kusek JW, Kanaya AM. Prevalence and risk factors for urinary incontinence in women with type 2 diabetes and impaired fasting glucose. Diabetes Care 2006;29(6):1307-12. https://doi.org/10.2337/dc05-2463 [Crossref]
