Fisioter Bras 2022;23(6):827-40
ORIGINAL ARTICLE
Effects of group physiotherapy on motor function in parkinsonism:
quasi-experimental study
Efeitos de
fisioterapia em grupo na função motora no parkinsonismo: estudo quasi-experimental
Aurelio de Melo Barbosa, M.Sc. Ft.*, Nathyele Oliveira
Fortaleza, Ft.**, Jordana Alves Castro, Ft.***, Roseane Assis Rio Branco
Bastos, Ft.****, Georgia Silva Menezes*****, Mayara Cordeiro de Faria******,
Flávia Martins Gervásio, Ft. D.Sc.*; Paola Ramos
Silva Neves, Ft. *
*Docente do curso de
Fisioterapia na Universidade Estadual de Goiás, Goiânia, GO, **Egressa da
Universidade Estadual de Goiás, Goiânia, GO, ***Residente no Hospital de
Urgência de Goiânia Otávio Lage, Goiânia, GO, ****Hospital Alberto Rassi, Goiânia, GO, *****Hospital Israelita Albert Einstein
Unidade Goiânia, Mestranda na Universidade Federal de Goiás, Goiânia, GO,
******Centro de Orientação, Reabilitação e Assistência ao Encefalopata
(CORAE), Goiânia, GO, Brazil
Received: August 30, 2022; Accepted:
September 30, 2022.
Correspondence: Aurelio de Melo Barbosa, Rua 26, 521
Jardim Santo Antonio 74853-070 Goiânia GO, Brazil
Aurelio de
Melo Barbosa: aurelio.barbosa@ueg.br
Nathyele Oliveira Fortaleza:
nathyelefortaleza2@hotmail.com
Jordana Alves
Castro: joalvescastro@gmail.com
Roseane
Assis Rio Branco Bastos: rose_arbbastos@outlook.com
Georgia
Silva Menezes: georgiasmenezess@gmail.com
Mayara
Cordeiro de Faria: maycfaria.fisio@gmail.com
Flávia
Martins Gervásio: flavia.gervasio@hotmail.com
Paola
Ramos Silva Neves: ft.paolarsn@gmail.com
Abstract
Objective: To study the effects of short-term group
physiotherapy protocols on motor functional variables in subjects with
Parkinsonism. Methods: A quasi-experimental, controlled, non-randomized,
open label study, with consecutive arms for one group (n = 6) and parallel arms
for the other (n = 9). One group (n = 9) observed by the researchers, underwent
group physical therapy once a week at a rehabilitation center for 5.5 to 7.5
months. Another group (n = 6) did not undergo physical therapy for a period of 5
months (control phase) and, consecutively, underwent group physical therapy
twice a week for 12 weeks. Both groups were assessed with the Berg Balance
Scale, Timed Up and Go Test, 5-Times Sit-to-Stand Test and 6-Minute Walk Test. Results:
In all the variables analyzed, there were no statistically significant
differences between the values measured in the assessments before and after the
interventions (physical therapy once or twice a week) and the control phase. Conclusion:
A short-term group physical therapy protocol, with a frequency of 1 or 2 times
a week, may not be enough to improve motor control people with Parkinsonism.
Keywords: muscle strength; postural balance; physical fitness;
physical therapy modalities; Parkinsonian disorders.
Resumo
Objetivo: Verificar os efeitos de protocolos de
fisioterapia em grupo, de curta duração, sobre variáveis funcionais motoras em
sujeitos com parkinsonismo. Métodos: Estudo quasi-experimental,
controlado, não randomizado, sem mascaramento, com braços consecutivos para um
grupo (n = 6) e paralelo para outro (n = 9). Um grupo (n = 9), observado pelos
pesquisadores, foi submetido a fisioterapia em grupo, uma vez por semana, em um
centro de reabilitação, durante 6,4 meses. Outro grupo (n = 6) não fez
fisioterapia por um período de 5 meses (fase controle) e, consecutivamente, foi
submetido a fisioterapia em grupo duas vezes por semana durante 12 semanas.
Ambos os grupos foram avaliados através da escala de equilíbrio de Berg, Teste Timed Up and
Go, Teste de sentar e levantar 5 vezes e Teste de caminhada de 6 minutos. Resultados:
Em todas as variáveis analisadas, não houve diferenças estatisticamente
significativas entre os valores mensurados nas avaliações antes e depois das
intervenções (fisioterapia 1 vez ou 2 vezes por semana) ou da fase controle. Conclusão:
Um protocolo de fisioterapia em grupo de curto prazo, com frequência de 1 ou 2
vezes por semana, talvez não seja suficiente para promover ganhos motores em
pessoas com parkinsonismo.
Palavras-chave: força muscular; equilíbrio postural;
aptidão física; modalidades de fisioterapia; transtornos parkinsonianos.
Introduction
Parkinson's disease (PD) is an idiopathic neurological
disorder that affects the substantia nigra and is the second most common
neurodegenerative disease, surpassed only by Alzheimer's disease [1]. It is
determined by neurobehavioral and/or musculoskeletal signs and symptoms:
rigidity, tremor, bradykinesia, stooped posture, postural instability, changes
in balance, gait, and muscle weakness [2]. Its classic diagnosis is based on
the presence of these clinical criteria, which are also used to examine and
classify the severity of PD, through the use of the Hoehn and Yahr Disability Stages Scale, which comprises five stages:
the first one has unilateral involvement with minimal or no functional
impairment and the last one presents severe motor impairment and total
dependence on mobility and self-care activities [2,3].
Parkinsonism is a term used to describe a syndrome
that has bradykinesia plus at least one of the following cardinal signs, as per
United Kingdom Parkinson's Disease Society Brain Bank (UKPDSBB) criteria:
tremor, rigidity, or postural instability. Primary parkinsonism corresponds to
PD, which has idiopathic cause, whereas secondary parkinsonism (SP) has
specific causes, usually other neurological diseases, or drug-induced injuries
[4].
People with PD who participate in exercise programs
show improvement in their motor functionality, with a better performance in
functional mobility activities, muscle strength, endurance, coordination,
flexibility, and balance when walking. This improve their quality-of-life
perception, when they are compared to physically inactive patients [3]. Group
physical therapy, performed with many patients at the same time under the
supervision of a professional, is important as a neurorehabilitation program in
the global management of PD, because it can improve balance, gait, and
performance of activities of daily living [5].
This study verified the effects of short-term and
group physical therapy protocols on motor functional variables in subjects with
Parkinsonism (PD or SP).
Methods
This is a quasi-experimental (observational with
therapeutic intervention), controlled, non-randomized, unblinded study, with a
consecutive arm for one group and parallel for another. The study followed the
recommendations of STROBE – STrengthening the
Reporting of OBservational studies in Epidemiology
(https://www.strobe-statement.org). The study was conducted between October
2017 and December 2018.
This investigation followed the Brazilian guidelines
and regulatory standards for research involving human beings and the Research
Ethics Committee of State University of Goias
approved it, under register 2.024.873/2017. Also, it was registered in the
Brazilian Registry of Clinical Trials (REBEC) under the digital identifier
(DI): RBR-392cxdp. All participants signed the informed consent after they were
informed about all aspects of the research.
Inclusion criteria
People who have PD or SP, diagnosed at least six months ago;
Be classified in stages 1, 2 or 3 of the Hoehn and Yahr
scale (HY) [6,7] or, in the case of SP, with a motor status equivalent to these
stages;
Be able to walk with total or modified independence;
Be aged between forty and seventy years;
Be under medical supervision for at least two months;
Have medical permission to practice exercises;
Make use of antiparkinsonian medication and have good adherence, with
regular use.
Exclusion criteria
People who have undergone surgery for PD;
Have cardiovascular diseases that contraindicate physical exercise;
Have rheumatological, orthopedic and traumatological diseases that
prevent joint mobility and contraindicate physical exercise;
Have arthrodesis in lower limbs and/or spine;
Have great limitation of joint movement in the lower and upper limbs and
spine;
Present secondary balance disorders, such as severe vestibular
disorders;
Have severe cognitive impairment, which prevents understanding of verbal
instructions. The Mini-Mental State Examination (MMSE) should confirm this:
with a score of less than 24 points for subjects with 8 years of schooling,
less than 18 points for those with less than 8 years of schooling; and less
than 14 points for illiterate people [8].
The intervention and control groups were organized as
follows:
OG-E1wI (observed group, exposed to the once-a-week intervention):
observed group, exposed to group physical therapy intervention once a week, each
session lasting 40 minutes. The exercises performed included flexibility
training, trunk and limb strengthening, motor coordination and gait. The
researchers did not treat this group, they only just observed (assessed) it.
Other physiotherapists treated this group at a public rehabilitation center in Goias. The researchers assessed every patient twice, with a
difference of 5.5 to 7.5 months (minimum and maximum time), with a mean of 6.4
months (193 days) between measurements. This, therefore, was an observational
group of exposure to a protective factor (group motor training).
EG-C (experimental group, controlled phase): experimental group, control
phase, monitored exclusively by the team of researchers at the State University
of Goias (SUG). The treatment and evaluations were
conducted by the researchers at the facilities of the Physiotherapy and
Physical Education College at SUG, in Goiania, Brazil. This group was assessed
three times over time. However, between the 1st and 2nd evaluation, the
participants were not exposed to motor training, they remained sedentary.
Therefore, the period between the 1st and 2nd assessment constitutes an
independent control group for the OG-E1wI and a paired control group for the
EG-2wI. This control period ranged from 3.7 to 6 months (minimum and maximum
time), with an average of 5 months (150 days).
EG-2wI (experimental group, twice a week intervention phase):
experimental group, intervention phase, monitored in the SUG, period that
corresponds to the time phase between the second and third assessment. During
this period, the participants did a group physiotherapy protocol, which lasted
twelve weeks, with two sessions per week, lasting fifty to sixty minutes each,
totaling twenty-four sessions. We made the second evaluation in the week before
the first week of treatment, and we performed the third evaluation three months
(ninety days) after the second evaluation, in the week after the last week of
treatment. The protocol included self-stretching and isometric exercises with
yoga and Pilates postures held for thirty seconds; trunk and limb strengthening
exercises, performed in an active-free or active-resisted way, followed by
circuit gait training with auditory cues.
There were two therapeutic arms:
Once-weekly treated arm: comparison of motor control evolution between
OG-E1wI (treated group) and EG-C (untreated control group), independent
samples;
Twice weekly treated arm: comparison between EG-2wI (treated group) and
EG-C (untreated control group), paired (dependent) samples.
Two researchers (GSM and MCF) blindly performed
assessments. The following assessment instruments were used:
Anamnesis;
Hoehn and Yahr Disability Stages Scale [6,7];
MMSE [8];
Timed Up and Go Test (TUG): measured time (TUG-t) and number of steps
(TUG-s) [9,10];
Berg Balance Scale (BBS) [11];
Five times sit and stand test (5T-SST) [12,13];
Right hand grip strength (RHGS) and left hand
grip strength (LHGS) measured with a Jamar® hydraulic dynamometer, according to
the American Society of Hand Therapists protocol, with three measurements and
considered the highest value in the dominant and non-dominant hand, with an
interval of 1 minute between each measurement [14,15];
The distance covered in 6-minute walk test (6MWT), performed according
to the recommendations of the American Thoracic Society [16].
In the statistical analysis, we used Excel® [17] to
tabulate the data and prepare the descriptive statistics. Muscle strength
(RHGS, LHGS and 5T-SST measurements), postural balance (BBS, TUG-t, TUG-s) and
endurance (6MWT) were the dependent variables in the statistical analysis. The
independent variables were the interventions. For inferential statistics, we
used the Action Stat Pro version 3.6 application [18].
As there are 5 measures (assessments) for each
dependent variable, there are 10 possible pairs of differences between measures
to be established, although only 3 pairs are relevant to the research:
difference between values of the 1st and 2nd assessment in the OG-E1wI,
difference between 1st and 2nd assessment values in EG-C and difference between
2nd and 3rd assessment values in EG-2wI. All samples of the dependent variables
showed non-normal distributions when analyzed with the Shapiro-Wil Test. For
this reason, we used the Wilcoxon Test with an unusual p-value (p = 0.005),
because it was necessary to divide the p-value by 10 to have a maximum alpha
error of 5%, considering the 10 possible comparisons between 5 samples of
collected data [19,20].
The study had great difficulty in recruitment and
adherence, with large sample losses, with a final sample of fifteen
participants. There was no guarantee of statistical power of 80% for the
statistical analyses.
Results
We recruited 50 participants, who signed the consent
form and were screened according to the inclusion and exclusion criteria. We
began the study with 41 volunteers who met all inclusion criteria. There were
26 sample losses at follow-up, with a final sample of 15 participants. The
figure 1 shows the flowchart of the intervention procedures performed in the
study.
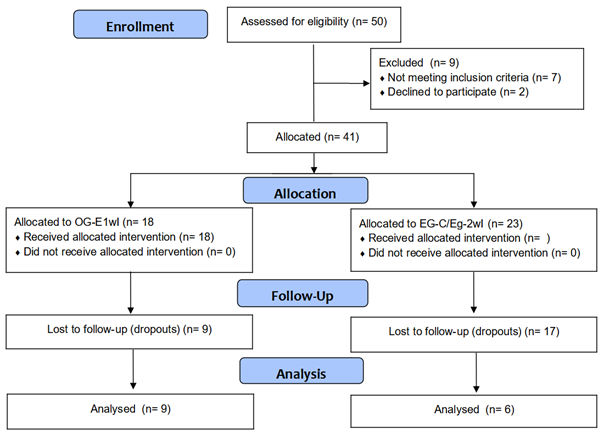
Figure 1 – Flowchart of intervention procedures
In the OG-E1wI, seven participants were male and two
were female. The age ranged from 44 years (minimum) to 77 years (maximum), with
mean and standard deviation of de ![]() = 62.3 (7.6) years. The BMI
(body mass index) ranged from 20.7 kg/m2 (minimum) to 32.7 kg/m2
(maximum), with mean and standard deviation of de
= 62.3 (7.6) years. The BMI
(body mass index) ranged from 20.7 kg/m2 (minimum) to 32.7 kg/m2
(maximum), with mean and standard deviation of de ![]() = 25.7 (3.4) kg/m2.
Still, three participants had SP and 6 PD, four of them in stage I of the HY
Scale, one participant in stage II and one participant in stage III.
= 25.7 (3.4) kg/m2.
Still, three participants had SP and 6 PD, four of them in stage I of the HY
Scale, one participant in stage II and one participant in stage III.
In the EG-C/EG-2wI, 2 participants were male and four
were female. The age ranged from 43 years (minimum) to 72 years (maximum), with
mean and standard deviation of ![]() =
62.8 (8.2) years. BMI ranged from 21.6 kg/m2 (minimum) to 32.0 kg/m2
(maximum), with mean and standard deviation of
=
62.8 (8.2) years. BMI ranged from 21.6 kg/m2 (minimum) to 32.0 kg/m2
(maximum), with mean and standard deviation of ![]() =
25.8 (2.4) kg/m2. Also, five participants had PD, all in stage I of
the HY Scale, and a participant had SP. This subject with SP and a participant
with PD had choreoathetosis secondary to the use of antiparkinsonian drugs.
=
25.8 (2.4) kg/m2. Also, five participants had PD, all in stage I of
the HY Scale, and a participant had SP. This subject with SP and a participant
with PD had choreoathetosis secondary to the use of antiparkinsonian drugs.
The results, obtained in the dependent variables in
each sample group, are shown in table I. The results of the differences between
the values obtained in the assessments conducted after and before the
intervention in each therapeutic arm are presented in table II.
Table II shows that there were no statistically significant
differences between the values obtained in the first and second assessments in
the OG-E1wI and EG-C and between the second and third assessments in the
EG-2wI.
Table I - Distributions of values of the variables in the
OG-E1wI and EG-C/EG-2wI
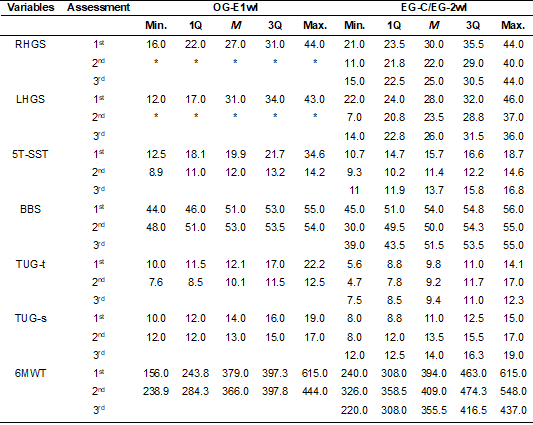
*In the OG-E1wI, in the second assessment, the
handgrip dynamometer was not available. So, we did not collect this
information. 1Q = Value of the first quartile; 3Q = Third quartile value;
5T-SST = five times sit and stand test; 6MWT = 6-minute walk test; BBS = Berg
Balance Scale; LHGS = Left handgrip strength; M = Median value; Max. = Maximum
value; Min. = Minimum value; RHGS = Right handgrip strength; TUG-s = Timed Up
and Go Test – steps (number of steps); TUG-t = Timed Up and Go Test – time
(time result, in seconds)
Table II - Distributions of differences in measurements of
variables in OG-E1wI, EG-C and EG-2wI
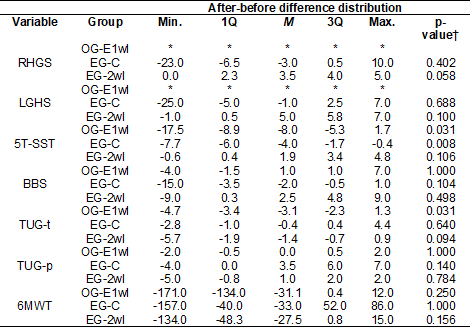
*In the OG-E1wI, in the second assessment, the
handgrip dynamometer was not available. So, we did not collect this
information. Thus, it was not possible to present the distribution of
after-before differences in this group. †Wilcoxon test p-value data. To be
statistically significant, the after-before difference should have a p-value of
0.005 or less (as explained in the methods section). 1Q = Value of the first
quartile; 3Q = Third quartile value; 5T-SST = five times sit and stand test;
6MWT = 6-minute walk test; BBS = Berg Balance Scale; LHGS = Left handgrip
strength; M = Median value; Max. = Maximum value; Min. = Minimum value; RHGS =
Right handgrip strength; TUG-s = Timed Up and Go Test – steps (number of
steps); TUG-t = Timed Up and Go Test – time (time result, in seconds)
Discussion
The aim of this study was to determine the effects of
a physical therapy protocol on the motor function of individuals with PD
classified as Hoehn Yahr stages 1, 2 and 3 or with
SP. The results suggest that exposure to a group kinesiotherapy intervention,
either twice a week or once a week, does not have important effects on muscle
strength, postural balance, and endurance capacity of the participants. We
emphasize that the reduced sample did not allow adequate statistical power to
detect differences.
However, we also hypothesized that the intervention
may not have had clinically important therapeutic effects on the patients’
motor control because the intervention was infrequent, with weekly frequencies
of once a week (in the OG-E1wI) and twice a week (in the EG-2wI).
Only 2 studies (Tollár et
al. [21,22], with treatment of 5 sessions per week for 3 weeks; Kurt et
al. [23], with 5 sessions per week for 5 weeks) found that group physical
therapy has effects on the dynamic postural balance, as measured by the TUG
test, with statistically significant differences between measurements after and
before treatment or in the mean difference between the group physical therapy
group and the control group without motor intervention. Another 5 clinical
trials (Hashimoto et al. [24], with treatment of 1 weekly session for 12
weeks; Hubble et al. [25,26], with 1 weekly session for 12 weeks; Claesson et al. [27], with 2 sessions per week for 8
weeks; King et al. [28], with 3 sessions per week for 4 weeks) found no
statistically significant difference between group physical therapy group and
no motor treatment group or even between values measured after and before group
physical therapy treatment. In the studies that demonstrated the effects of
group physical therapy on dynamic balance, the weekly frequency of
consultations was 5 times a week. In the other studies, in which physical
therapy had no effect, the frequencies ranged from 1 to 3 sessions per week.
All studies that measured static postural balance
found effects of group physical therapy. In the measurement through the BBS or
the MiniBESTest (Mini Balance Evaluation Systems
Test), 8 studies (Conradsson et al. [29],
treatment with 3 weekly sessions for 10 weeks; Sparrow et al. [30], 3
weekly sessions for 3 months ; Landers et al. [31], with 3 weekly
sessions for 8 weeks; Claesson et al. [27];
Hashimoto et al. [24]; Hubble et al. [25,26]; King et al.
[28] ; Kurt et al. [23]) reported statistically significant differences
between measurements after and before treatment or in the mean difference
between active and control groups. As for the measurement through stabilometry, the study by Tollár
et al. [21,22] found that group physical therapy was superior to
control, with a statistically significant reduction in body sway measurements.
Also, the Kurt et al. [23] trial revealed a statistically significant
reduction in body sway measurements, comparing values measured after and before
treatment.
The study by Lander et al. [31] demonstrated
that group physical therapy promotes a statistically significant increase in
the values of the 6MWT and in the muscular strength of the lower limbs measured
through the sit-and-stand test for 30 seconds (a variation of the 5T-SST).
The study by Sparrow et al. [30] reports that
group physical therapy, compared to no-treatment control, reduced the rate of
falls per month, with a reduction of 36.8% (rate ratio= 0.632 [0.524, 0.763]
95% CI).
Previously reported clinical studies used a variety of
motor training strategies, such as: 45 minutes of focused somatosensory balance
training without visual or audible cues [27]; the HiBalance
program, a 60-minute regimen of highly challenging balance training that
incorporates two-task and PD-specific balance components [29]; 60 minutes of
high intensity and agility exercises using sensorimotor and visual stimuli,
training mobility and postural balance [21,22]; ABC program (Agility Boot Camp)
with 60 minutes of activities that target basic postural systems in a “training
camp” model to target biomechanical restrictions, kinesthesia, stability
limits, anticipatory postural adjustments, bradykinesia and coordination during
gait, with 6 stations (Tai chi, Boxing, Lunges, Kayaking, Agility Course and
Pilates) with activities that progress in difficulty [28]; 90-minute program of
strengthening exercises, range of motion, reactive and anticipatory balance
activities, sensory input alteration and gait training [30]; training camp with
90 minutes of high-intensity exercises (aerobic, resistance, balance and
flexibility) [31]; 60-minute warm-up training program, stretching, joint
mobilization and gait exercises [23]; 90 minutes of trunk mobility and
endurance training [25,26]; 60 minutes of flexibility, balance, postural
transfer (from sitting to standing and vice versa, repeatedly) and gait
training [24].
The present study has limitations: the small number of
participants due to large sample losses, which may have caused lower
statistical power of the investigation. In order to obtain more reliable
conclusions about the effects of group physical therapy on the motor control of
people with parkinsonism, it is necessary to conduct randomized clinical trials
with a larger sample and longer follow-up time to verify the effects on motor
control variables of group physical therapy delivered one or two times a week.
Conclusion
The data from the present study suggest that a group
physical therapy protocol with a frequency of 1 or 2 times a week, training
flexibility, strength (training with light to moderate load) and gait, may not
be enough to improve motor control in people with Parkinson Disease in stages
1-3 or with mild to moderate SP.
The scientific literature data suggest that group
physical therapy with a greater weekly frequency, of at least three weekly
sessions, has effects of improvement in motor control, especially protocols
with five weekly sessions and longer session duration.
Conflicts of interest
The authors declare no
conflicts of interest.
Funding sources
The authors declare no
funding sources.
Authors' contributions
Research conception and
design: Barbosa AM, Gervasio FM; Data collection: Barbosa AM, Fortaleza
NO, Castro JA, Bastos RARB (intervention in the experimental group); Menezes
GS, Faria MC (physiotherapist’s assessments); Data analysis and
interpretation: Barbosa AM, Fortaleza NO, Castro JA, Gervasio FM, Menezes
GS, Faria MC, Neves PRS, Bastos RARB; Statistical analysis: Barbosa AM;
Writing of the manuscript: Barbosa AM, Fortaleza NO, Neves PRS; Critical
review of the manuscript for important intellectual content: Barbosa AM,
Fortaleza NO, Castro JA, Bastos RARB, Menezes GS, Faria MC, Gervasio FM, Neves
PRS
- Hayes MT. Parkinson’s Disease and Parkinsonism. Am J Med 2019;132:802-7. doi: 10.1016/J.AMJMED.2019.03.001 [Crossref]
- Cabreira V, Massano J. Doença de Parkinson: Revisão clínica e atualização. Acta Med Port 2019;32:661-70. doi: 10.20344/AMP.11978 [Crossref]
- Azevedo IM, Gondim ITGO, Silva KMC, Oliveira CA, Lins CC dos SA, Coriolano M das GWS. Repercussões da estimulação auditiva rítmica sobre a funcionalidade na doença de Parkinson. Fisioter Mov 2021;34:1-2. doi: 10.1590/FM.2021.34116 [Crossref]
- Barbosa MT. Diagnóstico e
tratamento da doença de Parkinson em idosos. In: Sociedade Brasileira de
Geriatria e Gerontologia, Cabrera M, Cunha U, editors.
PROGER Programa Atualização em Geriatria e Gerontologia. Ciclo 7, Porto Alegre: Artmed; 2021. p.9-52.
- Clementino ACCR, Menezes DSP, Guimarães DGG, Fernandes GN, Silva ASA, Menezes DMP, et al. Influence of group physiotherapy treatment on mobility, balance and quality of life in people with Parkinson’s disease. Brazilian J Dev 2022;8:9322-44. doi: 10.34117/BJDV8N2-060 [Crossref]
- Goulart F, Pereira LX, Goulart DF. Uso de escalas para avaliação da doença de Parkinson em fisioterapia. Fisioter Pesqui 2005;11:49-56. doi: 10.1590/FPUSP.V11I1.76385 [Crossref]
- Mello MPB, Botelho ACG. Correlação das escalas de avaliação utilizadas na doença de Parkinson com aplicabilidade na fisioterapia. Fisioter Mov 2010;23:121-7. doi: 10.1590/S0103-51502010000100012 [Crossref]
- Bertolucci PHF, Brucki SMD, Campacci SR, Juliano Y. O Mini-Exame do Estado Mental em uma população geral: impacto da escolaridade. Arq Neuropsiquiatr 1994;52:01-7. doi: 10.1590/s0004-282x1994000100001 [Crossref]
- Christofoletti G, Freitas RT, Cândido ER, Cardoso CS. Eficácia de tratamento fisioterapêutico no equilíbrio estático e dinâmico de pacientes com doença de Parkinson. Fisioter Pesqui 2010;17:259-63. doi: 10.1590/S1809-29502010000300013 [Crossref]
- Santos FPV, Borges LL, Menezes RL. Correlação entre três instrumentos de avaliação para risco de quedas em idosos. Fisioter Mov 2013;26:883–94. doi: 10.1590/S0103-51502013000400017 [Crossref]
- Bertoldi FC, Silva JAMG, Faganello-Navega FR. Influência do fortalecimento muscular no equilíbrio e qualidade de vida em indivíduos com doença de Parkinson. Fisioter Pesqui 2013;20:117-22. doi: 10.1590/S1809-29502013000200004 [Crossref]
- Nakano MM. Versão brasileira da Short Physical Performance Battery SPPB: adaptação cultural e estudo da confiabilidade. Campinas: Universidade Estadual de Campinas, 2007. doi: 10.47749/T/UNICAMP.2007.396756 [Crossref]
- Antônio AM, Antônio AM dos S, Bertoldi FC, Faganello-Navega FR. Influência do fortalecimento muscular na independência funcional de indivíduos parkinsonianos. ConScientiae Saúde 2013;12:439-46. doi: 10.5585/conssaude.v12n3.4376 [Crossref]
- Caporrino FA, Faloppa
F, Santos JBG, Réssio C, Soares FHC, Nakachima LR, et al. Estudo populacional da força de
preensão palmar com dinamômetro Jamar®. Rev
Bras Ortop [Internet] 1998[cited 2022 Nov 14];33:150-4. Available from:
https://cdn.publisher.gn1.link/rbo.org.br/pdf/33-2/1998_fev_04.pdf
- Richards LG, Olson B, Palmiter-Thomas P. How forearm position affects grip strength. Am J Occup Ther 1996;50:133-8. doi: 10.5014/AJOT.50.2.133 [Crossref]
- Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, et al. ATS Statement 2012;166:111-7. doi: 10.1164/AJRCCM.166.1.AT1102 [Crossref]
- Microsoft Corporation. Microsoft 365 Excel 2018.
- Grupo EstatCamp. Action Stat Pro® 2017.
- Almeida CO. Ensaios
da amostra ao teorema do limite central: um pouco dos fundamentos e uma
aplicação prática. Cruz Das Almas (BA): Embrapa Mandioca e Fruticultura; 2019.
- Rumsey DJ. Estatística II para leigos. Rio de
Janeiro: Alta Books; 2014.
- Tollár J, Nagy F, Kovács N, Hortobágyi T. A high-intensity multicomponent agility intervention improves parkinson patients’ clinical and motor symptoms. Arch Phys Med Rehabil 2018;99:2478-2484.e1. doi: 10.1016/j.apmr.2018.05.007 [Crossref]
- Tollár J, Nagy F, Kovács N, Hortobágyi T. Two-year agility maintenance training slows the progression of parkinsonian symptoms. Med Sci Sports Exerc 2019;51:237-45. doi: 10.1249/MSS.0000000000001793 [Crossref]
- Kurt EE, Büyükturan B, Büyükturan Ö, Erdem HR, Tuncay F. Effects of Ai Chi on balance, quality of life, functional mobility, and motor impairment in patients with Parkinson’s disease. Disabil Rehabil 2018;40:791-7. doi: 10.1080/09638288.2016.1276972 [Crossref]
- Hashimoto H, Takabatake S, Miyaguchi H, Nakanishi H, Naitou Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: a quasi-randomized pilot trial. Complement Ther Med 2015;23:210-9. doi: 10.1016/J.CTIM.2015.01.010 [Crossref]
- Hubble RP, Naughton G, Silburn PA, Cole MH. Trunk exercises improve gait symmetry in Parkinson disease: a blind phase II randomized controlled trial. Am J Phys Med Rehabil 2018;97:151-9. doi: 10.1097/PHM.0000000000000858 [Crossref]
- Hubble RP, Silburn PA, Naughton GA, Cole MH. Trunk exercises improve balance in Parkinson disease: A phase II randomized controlled trial. J Neurol Phys Ther 2019;43:96-105. doi: 10.1097/NPT.0000000000000258 [Crossref]
- Claesson IM, Ståhle A, Lökk J, Grooten WJA. Somatosensory Focused Balance Training without cues can improve balance and gait in early Parkinson’s disease – a randomised pilot study. Eur J Physiother 2017;20:67-73. doi: 10.1080/21679169.2017.1363284 [Crossref]
- King LA, Wilhelm J, Chen Y, Blehm R, Nutt J, Chen Z, et al. Effects of group, individual, and home exercise in persons with Parkinson disease. J Neurol Phys Ther 2015;39:204-12. doi: 10.1097/NPT.0000000000000101 [Crossref]
- Conradsson D, Löfgren N, Nero H, Hagströmer M, Ståhle A, Lökk J, et al. The effects of highly challenging balance training in elderly with Parkinson’s disease: A randomized controlled trial. Neurorehabil Neural Repair 2015;29:827-36. doi: 10.1177/1545968314567150 [Crossref]
- Sparrow D, Angelis TR, Hendron K, Thomas CA, Saint-Hilaire M, Ellis T. Highly challenging balance program reduces fall rate in Parkinson disease. J Neurol Phys Ther 2016;40:24-30. doi: 10.1097/NPT.0000000000000111 [Crossref]
- Landers MR, Navalta JW, Murtishaw AS, Kinney JW, Pirio Richardson S. A High-intensity exercise boot camp for persons with Parkinson disease: a phase II, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther 2019;43:12-25. doi: 10.1097/NPT.0000000000000249 [Crossref]
