Fisioter Bras. 2023;24(1):27-41
ORIGINAL ARTICLE
Influence of body composition during hospitalization on lung function
and inspiratory muscle strength in patients with COVID 19: cross-sectional
study
Influência da
composição corporal durante a hospitalização sobre a função pulmonar e força da
musculatura inspiratória em pacientes com COVID-19: estudo transversal
Juliano Giorgio Rosa Luccas1,
Larissa de Magalhães Doebeli Matias1, Hiago Vinicius Costa Silva1, Charlys
Victor Sousa Aguiar1, João Paulo Rodrigues Pacheco1,
Everton Teles Rodrigues1, Juliana Ribeiro Fonseca Franco de Macedo2,
Adriana Claudia Lunardi3,4, Elinaldo da
Conceição dos Santos1
1Universidade Federal do Amapá, Brazil
2Université Catholique
de Louvain, Belgium
3Universidade Cidade de São Paulo, São
Paulo, Brazil,
4Universidade de São Paulo, São Paulo, Brazil
Received: November 12,
2022; Accepted: January 20,
2023.
Correspondence: Elinaldo da
Conceição dos Santos, e-mail: drelinaldo@yahoo.com.br
How to
cite
Luccas JGR, Matias LMD, Silva
HVCS, Aguiar CVS, Pacheco JPR, Rodrigues ET, Macedo JRFF, Lunardi
AC, Santos EC. Influence of body composition during hospitalization
on lung function and inspiratory muscle strength in patients with COVID 19:
cross-sectional study. Fisioter Bras. 2023;24(1):27-41. doi: 10.33233/fb.v24i1.5345
Abstract
Introduction: Approximately 31% of hospitalized patients with
COVID-19 lose more than 5% of their initial weight, leading to muscle weakness.
Therefore, body composition has become the focus of investigation, to estimate
pulmonary impairment, inspiratory muscle strength, and mortality. Objectives:
To investigate whether weight loss and body composition of patients
hospitalized with COVID-19 have any influence on lung function and inspiratory
muscle strength after hospital discharge. Methods: Cross-sectional
study. Adult patients were assessed after hospitalization due to COVID-19.
Outcomes evaluated were lung function, maximal inspiratory pressure (MIP), body
composition, and mortality. Correlations between the variables were estimated
by Pearson's Correlation Coefficient. Results: Forced Vital Capacity
(FVC) was correlated with weight loss, skeletal muscle mass, lean mass, left
leg, and fat-free mass; Forced expiratory volume in the first second (FEV1) was
correlated only with weight loss; and MIP was correlated with skeletal muscle
mass, lean mass, left leg, right leg, and fat-free mass. Conclusion: A
moderate correlation was observed between FVC and the analyzed body composition
variables, except lean mass of left leg; between FEV1 and weight loss; and
between MIP and the analyzed body composition variables, except weight loss.
Keywords: COVID-19; body composition; respiratory function
test.
Resumo
Introdução: Cerca de 31% dos pacientes
hospitalizados com COVID-19 perdem mais de 5% de seu peso inicial, levando a
fraqueza muscular. Portanto, a composição corporal tornou-se foco de
investigação, para estimar comprometimento pulmonar, força da musculatura
inspiratória e mortalidade. Objetivos: Investigar se a perda de peso e a
composição corporal de pacientes internados com COVID-19 influenciam na função
pulmonar e na força muscular inspiratória após alta hospitalar. Métodos:
Estudo transversal. Pacientes adultos foram avaliados após internação por
COVID-19. Os desfechos avaliados foram função pulmonar, pressão inspiratória
máxima (Pimáx), composição corporal e
mortalidade. As correlações entre as variáveis foram estimadas pelo Coeficiente
de Correlação de Pearson. Resultados: A capacidade Vital Forçada (CVF)
foi correlacionada com perda de peso, massa muscular esquelética, massa magra,
perna esquerda e massa livre de gordura; o volume expiratório forçado no
primeiro segundo (VEF1) correlacionou-se apenas com a perda de peso; e a Pimáx foi correlacionada com massa muscular
esquelética, massa magra, perna esquerda, perna direita e massa livre de
gordura. Conclusão: Observou-se correlação moderada entre CVF e as
variáveis de composição corporal analisadas, exceto massa magra da perna
esquerda; entre VEF1 e perda de peso; e entre Pimáx
e as variáveis de composição corporal analisadas, exceto perda de peso.
Palavras-chave: COVID-19; composição corporal; testes
de função respiratória.
Introduction
Coronavirus disease (COVID-19) is related to severe
and non-severe symptoms, such as dyspnea, cough, myalgia, asthenia, fever,
headache, and muscle weakness, and can negatively affect body weight [1].
Impaired body composition through weight loss impacts the course of the disease
and affects muscles and adipose tissues, making patients even more fragile and
worsening these symptoms [2,3]. Among hospitalized patients, underweight
patients have higher mortality rates [4]. Within 60 days after hospital discharge
9.1% of patients died, especially older adults [5].
Approximately 31% of hospitalized patients with
COVID-19 lose more than 5% of their initial weight, increasing the length of
the illness and hospital stay, leading to muscle weakness [1,6]. After hospital
discharge, this muscle weakness persists in 92% of patients [6]. During the
course of the disease, symptoms are often persistent and impactful, and muscle
weakness seems to impair lung function and maximal inspiratory pressure (MIP)
[7,8,9].
Considering the weight loss of patients hospitalized
with COVID-19, body composition has become the focus of investigation, using
bioelectrical impedance analysis (BIA), to estimate pulmonary impairment,
inspiratory muscle strength, and mortality [10]. However, there is still
uncertainty about the association between the patient's body composition after
hospital discharge and these variables. In addition, approximately 80% of
patients diagnosed with COVID-19 and hospitalized with clinical worsening, present
alterations in lung function at the time of hospital discharge, which can
predict the outcome of the COVID-19 disease [11,12]. Among these alterations,
it is possible to observe a decrease in FVC and MIP [11,13].
It is probable that adequate body composition can
improve clinical outcomes of the disease [14], such as lung function and MIP,
and reduce the mortality rate. Some studies have investigated the occurrence of
weight loss in patients with COVID-19 at different times; before, during, and after
hospital treatment [13,15,16], suggesting the possibility that patients with
COVID-19 may be susceptible to weight loss [3]. However, to date, no studies
have been published to clarify whether there is an association between body
composition and lung function, respiratory muscle strength, and mortality after
hospital discharge in patients with COVID-19.
The current cross-sectional study was designed to
answer the following question: - Does the weight loss and body composition
(skeletal muscle mass, lean mass, lean leg mass, fat-free mass) of patients
hospitalized with COVID-19 have any influence on lung function and inspiratory
muscle strength after hospital discharge?
Methods
Design
This study is a cross-sectional study conducted from
March to November 2021. The reporting followed the guidelines of the
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE
statement), using the specific extension for cross-sectional studies [17].
Ethical data
This study was conducted following the principles of
the Declaration of Helsinki: CAAE 40801920.80000.003.
Eligibility criteria
Adult patients post hospitalization (within 48 hours
after discharge) due to COVID-19 infection with undetectable viral load (after
hospital discharge), without previous chronic respiratory diseases from 4
reference centers for the treatment of COVID-19 in the city of Macapá (Brazil), and who lost weight during the hospital
stay (at least 1 kg), were included. Patients with a history of
endotracheal intubation during the hospital stay and any patients who wanted to
withdraw from participation in the study, were excluded.
Outcomes evaluated
Spirometry, manovacuometry,
and BIA were used to assess the outcomes. All tests were performed on the same
day, always in the morning. The test execution sequence was randomized for each
patient by drawing lots, aiming to minimize the overlapping effects. The random
draw was performed using dice, where values “1” and “2” represented spirometry,
“3” and “4” represented manovacuometry, and “5” and
“6”, the BIA.
1. Lung function was evaluated by spirometry (Spirobank
II, MIR, Italy) which was performed following international guidelines [18].
The variables FVC, FEV1, and PEF were analyzed. In addition, the predicted values
were recorded based on the Brazilian population [19].
2. MIP (measured in millimeters of mercury (mmHg)) was obtained by means
of a Wika manovacuometer
(WIKA, Ind, Ipero, SP, Brazil). The tests were
carried out according to the guidelines i.e., the maneuvers were performed
three to five times, from the residual volume, with the highest value used for
analysis [20,21]. The measurement was considered valid if three acceptable and
two reproducible measures were obtained [21].
3. Body composition: Weight loss was calculated based on the weight
recorded at the beginning and at the end of the hospital stay, using data from
the patient medical record. Skeletal muscle mass, lean mass, lean leg mass, and
fat-free mass were evaluated using the BIA InBody 770
device (InBody 770, Cerritos, CA, USA). The InBody 770 uses 30 impedance measurements and six different
frequencies. Participants were instructed to be present in the laboratory after
fasting for at least three hours and not to perform any exercise on the day of
assessment. All tests were conducted in the morning. For the analysis in the InBody 770, the individuals stood barefoot on the platform
of the device with the plantar regions on the electrodes and held the unit
handles with the thumb and fingers to maintain direct contact with the
electrodes. They remained standing for approximately one minute, keeping the
elbows fully extended and the shoulder joint abducted at an angle of
approximately 30 degrees.
4. Mortality after hospital discharge: the mortality rate was recorded
through the number of deaths. Patients were monitored via telephone, over a
3-month period, to observe and record this outcome, in order to establish
whether this variable is related to the course of the disease. Phone calls were
made once a week.
Statistical analysis
The sample size calculation was based on the Luiz
& Magnanini formula [22], assuming an α of
95%, type I error of 5%, and mortality rate 1.3% [23]. A minimum sample size of
20 patients was obtained. Considering possible losses to follow-up for any
reason, the sample was increased by 20%, giving a final sample of 25 patients.
For dichotomous variables, descriptive statistics were used to describe the
baseline. Variables are presented as absolute number and percentage (n (%)) and
mean and standard deviation (mean ± standard deviation). The D'Agostino Pearson
test was used to test data normality when necessary. The degrees of correlation
between the lung function data (FEV1, FVC, and PEF) and body composition, and
between the MIP data and body composition were estimated by Pearson's
Correlation Coefficient, considering a null correlation when r = 0 (zero), weak
correlation when 0 < r ≤ |0.3|, moderate correlation when |0.3| < r
≤ |0.6|, strong correlation when |0.6| < r ≤ |0.9|, very strong
correlation when |0.9| < r < |1|, and perfect correlation when r = 1
[24]. All statistical analyses were performed using BioEstat
5.3 software (Belém, Pará, Brazil) [25].
Results
During the data collection period, 27 patients were
eligible for the study, two of whom were excluded (one patient did not agree to
continue in the study and another reported that he had not been hospitalized,
but had simply remained in the hospital for a short period only for
observation) (figure 1).
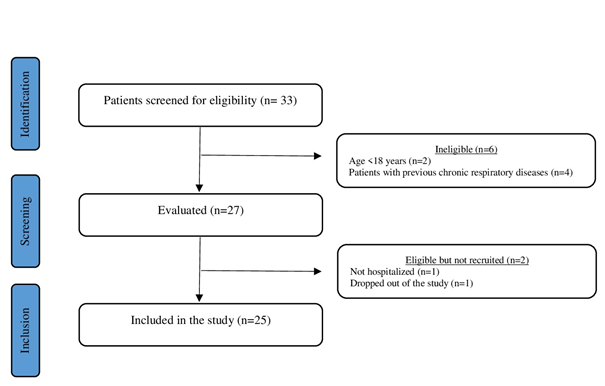
Figure 1 - Flowchart of the study design
Twenty-five patients previously infected with the COVID-19 virus and not
treated with invasive mechanical ventilation were
included in this study. The majority of patients were female, non-smoking, and
did not require non-invasive ventilation (NIV) during hospitalization. All
patients presented weight loss, ranging from 2 to 23 kg. The length of hospital
stay was 15 ± 9.6 days and the most frequently reported symptoms were dyspnea,
cough, ageusia, anosmia, and headache (table I). FVC, FEV1, and MIP variables
that demonstrated a statistically significant degree of correlation with body
composition variables were classified as "moderate correlation" (table
II and table III).
Table I - Patients’ characteristics and clinical
information
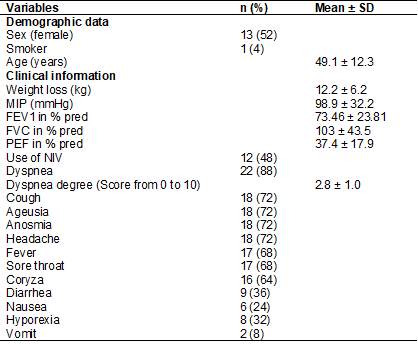
N = absolute number; SD = standard deviation; kg =
kilogram; NIV = non-invasive ventilation; MIP = maximal inspiratory pressure;
mmHg = millimeters of mercury; FEV1 = forced expiratory volume in one second;
FVC = forced vital capacity; PEF = peak of expiratory flow
Table II - Correlation of weight loss versus pulmonary
function and maximal inspiratory pressure
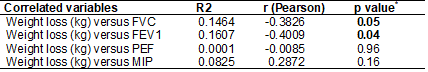
R2 = Determination coefficient; R (Pearson) = measure
of degree and direction by the linear correlation coefficient r; p value* = p
value of F (regression); kg = kilogram. FVC=forced vital capacity; FEV1=forced
expiratory volume in one second; PEF = peak expiratory flow; MIP = maximal
inspiratory pressure
Table III - Correlation of BIA versus Lung Function and MIP
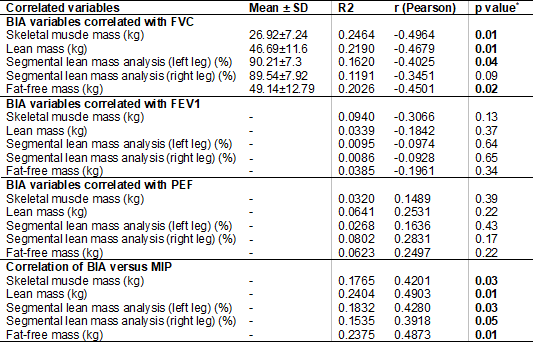
BIA = bioelectrical impedance analysis; MIP = maximal
inspiratory pressure; SD = standard deviation; R2 = Determination coefficient;
R (Pearson) = measure of degree and direction by the linear correlation
coefficient r; p value* = p value of F (regression); FVC = forced vital
capacity; FEV1=forced expiratory volume in one second; PEF = peak expiratory
flow; kg = kilogram; % = percentage
Evaluation of the relationship between lung function,
MIP, and body composition
Among the variables investigated, five presented a statistically
significant negative correlation with FVC: weight loss, skeletal muscle mass,
lean mass, lean mass of the left leg, and fat-free mass. Weight loss also
presented a statistically significant negative correlation with FEV1 (figure
2). These same variables had a positive correlation with MIP, except weight
loss (figure 3). No BIA variable was correlated with PEF (table III).
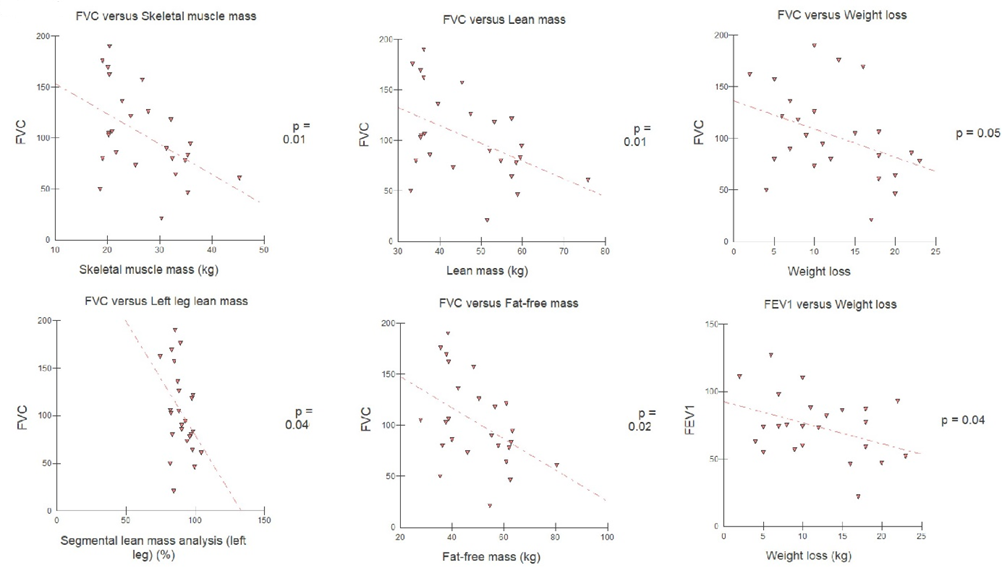
Figure 2 - Pearson's linear correlation between body
composition and FVC and FEV1
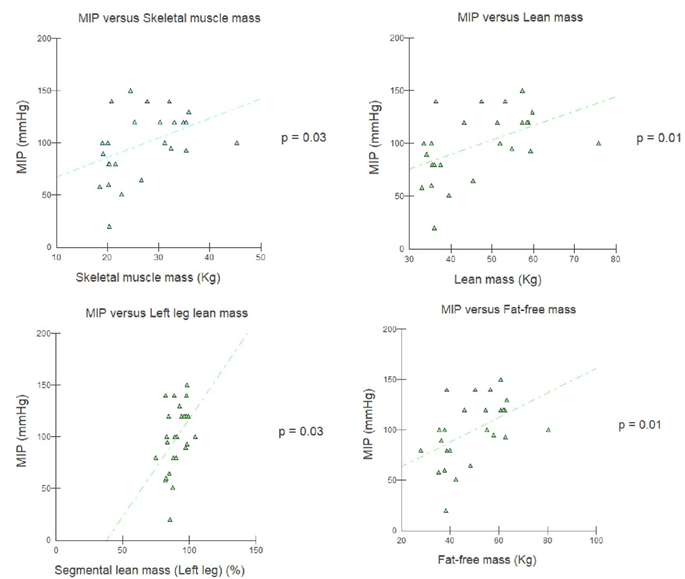
Figure 3 - Pearson's linear correlation between body
composition and MIP
Mortality rate after hospital discharge
There was no record of death after hospital discharge
among the investigated patients.
Discussion
The results of this cross-sectional study, including
twenty-five patients after hospital discharge to treat COVID-19 infection,
showed that FVC and MIP were associated with body composition (FVC was only not
associated with lean mass of the right leg), FEV1 was associated only with
weight loss, and PEF was not associated with any variables.
Patients hospitalized due to COVID-19 present impaired
lung function, with a reduction in FVC, FEV1, and inspiratory muscle strength
[26]. Of the total, 49.1% of patients had an MIP lower than 80% of the
predicted, which remained compromised in the period of convalescence from the
disease [27]. It is likely that body composition has some influence on these
effects, but further investigation is required. In addition, after hospital
discharge, approximately 80% of patients presented maintained impairment for a
long time, requiring long periods of clinical follow-up and respiratory
rehabilitation [11,26].
A study with 34 post-COVID-19 patients observed that
patients who lost muscle mass showed a statistically significant correlation
between skeletal muscle mass and FVC and FEV1 (0.582 and 0.592, respectively)
[28]. Park et al. [29] demonstrated that low skeletal muscle mass is
associated with a potential risk for pulmonary dysfunction, regardless of age
and sex.
This can be explained by the fact that low skeletal
muscle mass affects physical performance and functionality, negatively
impacting patients' physiology and metabolism [30,31], especially in patients
who remain in hospital for more than 20 days, losing lung volume and capacity,
which can lead to obstructive and restrictive ventilation disorders [32].
An experimental study investigated significant
alterations in body composition in twelve oxygen-dependent patients
hospitalized for 13.5 days (ranging from 8.3 to 27.8 days) with symptoms
present 4.5 days before admission due to COVID-19 infection. It was observed
that active metabolic muscle tissue and intracellular hydration status were
altered during the initial active phase of the infection, slowly recovering
until hospital discharge.
In addition, the literature points out that body
composition is associated with the evolution of COVID-19 [10], leading to more
severe symptoms and greater respiratory impairment. Our findings also indicate
that the rate of segmental fat in the trunk was not associated with any
respiratory functional variable investigated. On the other hand, the lack of an
association between the severity of the disease and body composition has been
reported, raising the possibility that other factors may interfere in the
course of the disease, with greater or lesser severity [33].
Experimental studies with adequate sample sizes are
needed to confirm whether body composition influences respiratory outcomes, so
that guidance can be given for the inclusion of nutritional support in the
treatment of patients with COVID-19 [3]. Thus, early identification of these
changes in body composition could help to direct more appropriate actions and
minimize the impact of COVID-19 in the patient's life [34].
Study limitations: (i) Lack
of nutritional biochemical assessment of patients included in the study, for
example, levels of potassium (K), sodium (Na), calcium (Ca), and magnesium
(Mg). Currently, in our hospitals, there is no routine assessment of the
nutritional biochemistry of patients with COVID 19. This study may point to the
need to perform this type of assessment on a daily basis to help answer
important clinical questions; (ii) We consider that the sample size of our
study was small and a future study should be performed with a larger sample
size.
Conclusion
The findings of this study enable us to conclude that
there is a negative association between FVC and the analyzed body composition
variables, except lean mass of left leg, and between FEV1 and weight loss, a
positive association between MIP and the analyzed body composition variables,
except weight loss. None of the variables were associated with PEF. These
findings are very important so that future experimental studies can investigate
the effects of body composition on the recovery of patients with COVID-19. We
also suggest studies with a larger sample size to confirm these findings and to
more accurately investigate the mortality of patients recovering from sequelae
of COVID-19 in the outpatient period.
Conflict of interests
The authors declare no conflict of interest.
Financing source
None
Author’s contributions
Conception and design of the study: Luccas JGR, Aguiar CVS, Santos EC;
: Matias LMD, Silva HVC, Aguiar CVS, Pacheco JPR,
Rodrigues ET; Data analysis and interpretation: Luccas
JGR, Matias LMD, Macedo JRFF, Lunardi AC, Santos EC; Statistical analysis: Luccas JGR, Lunardi AC, Santos EC; Manuscript writing:
Luccas JGR, Lunardi AC, Santos EC; Critical review
of the manuscript for important intellectual content: Lunardi AC; Santos EC
References
- Di Filippo L, De Lorenzo R,
D'Amico M, Sofia V, Roveri
L, Mele R, et al. COVID-19 is associated with clinically significant
weight loss and risk of malnutrition, independent of hospitalisation:
A post-hoc analysis of a prospective cohort study. Clin Nutr.
2021;40(4):2420-6. doi: 10.1016/j.clnu.2020.10.043 [Crossref]
- Gao M, Wang Q, Piernas C, Astbury NM, Jebb SA, Holmes MV, et al. Associations between body composition, fat distribution and metabolic consequences of excess adiposity with severe COVID-19 outcomes: observational study and Mendelian randomisation analysis. Int J Obes. 2022;46:943-50. doi: 10.1038/s41366-021-01054-3 [Crossref]
- Anker MS, Landmesser U, Haehling SV, Butler J, Coats AJS, Anker SD. Weight loss, malnutrition, and cachexia in COVID-19: facts and numbers. Journal of Cachexia, Sarcopenia and Muscle. 2021;12(1):9-13. doi: 10.1002/jcsm.12674 [Crossref]
- Albarrán-Sánchez A, Ramírez-Rentería C, Anda-Garay JC, Noyola-García ME, Alberti-Minutti P, Flores-Padilla G, et al. Differences in mortality rate among patients hospitalized with severe COVID-19 according to their body mass index. Obes Sci Pract. 2021;8(4):423-32. doi: 10.1002/osp4.584 [Crossref]
- Donnelly JP, Wang XQ, Iwashyna TJ, Prescott HC. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA. 2020;325(3):304-6. https://doi:10.1001/jama.2020.21465 [Crossref]
- Ballvé A, Llauradó A, Palasí A, Quintana M, Martínez-Sáez E, Laínez E, et al. Weakness as a complication of COVID-19 in critically ill patients: clinical features and prognostic factors in a case series. Rev Neurol. 2021;73(1):10-16. doi: 10.33588/rn.7301.2021042 [Crossref]
- Plaza M, Sevilla GGP. Respiratory muscle sequelae in young university students infected by coronavirus disease 2019: an observational study. Rev Assoc Med Bras. 2022;68(2):245-49. doi: 10.1590/1806-9282.20211040 [Crossref]
- Zhang S, Bai W, Yue J, Qin L, Zhang C, Xu S, et al. Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Scientific Reports. 2021;13854:1-13. doi: 10.1038/s41598-021-93191-y [Crossref]
- Carod-Artal FJ. Post-COVID-19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol. 2021;72(11):384-96. doi: 10.33588/rn.7211.2021230 [Crossref]
- Freuer D, Linseisen J, C. Meisinger. Impact of body composition on COVID-19 susceptibility and severity: A two-sample multivariable Mendelian randomization study. Metabolism. 2021;118:154732. doi: 10.1016/j.metabol.2021.154732 [Crossref]
- Polese J, Sant'Ana L, Moulaz IR, Lara IC, Bernardi JM, Lima MD, et al. Pulmonary function evaluation after hospital discharge of patients with severe COVID-19. Clinics. 2021;76:e2848. doi: 10.6061/clinics/2021/e2848 [Crossref]
- Morgenthau AS, Levin MA, Freeman R, Reich DL, Klang E. Moderate or severe impairment in pulmonary function is associated with mortality in sarcoidosis patients infected with SARS-CoV-2. Lung. 2020;198:771-75. doi: 10.1007/s00408-020-00392-9 [Crossref]
- Mancuzo EV, Marinho CC, Machado-Coelho GLL, Batista AP, Oliveira JF, Andrade BH, et al. Lung function of patients hospitalized with COVID-19 at 45 days after hospital discharge: first report of a prospective multicenter study in Brazil. J Bras Pneumol. 2021;47(6):e20210162. doi: 10.36416/1806-3756/e20210162 [Crossref]
- Thomas M, OJ Price, JH Hull. Pulmonary function and COVID-19. Physiol Curr Opin. 2021;21:29-35. doi: 10.1016/j.cophys.2021.03.005 [Crossref]
- Allard L, E Ouedraogo, Molleville J, Bihan H,Giroux-Leprieur B, Sutton A, et al. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020;12(12):E3679. doi: 10.3390/nu12123679 [Crossref]
- Pironi L, Sasdelli AS, Ravaioli F, Baracco B, Battaiola C, Bocedi G, et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2021;40(3):1330-7. doi: 10.1016/j.clnu.2020.08.021 [Crossref]
- Elm EV, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-9. doi: 10.1016/j.jclinepi.2007.11.008 [Crossref]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-38. doi: 10.1183/09031936.05.00034805 [Crossref]
- Sociedade Brasileira de
Pneumologia e Tisiologia. Diretrizes para Testes de Função Pulmonar. J Pneumol [Internet]. 2002 [cited 2023 Jan 13];28(Suppl 3):S1-S238. Available from :
https://www.jornaldepneumologia.com.br/details-supp/45
- American Thoracic Society/European Respiratory. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518-24. doi: 10.1164/rccm.166.4.518 [Crossref]
- Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32(6):719-27. doi: 10.1590/S0100-879X1999000600007 [Crossref]
- Thluiz RR, Magnanini
MMF. The logic of sample determination in epidemiological research. Cad Health
Collect [Internet]. 2000 [cited 2023 Jan 13];8(2):9-28. Available from:
https://pesquisa.bvsalud.org/portal/resource/pt/lil-326604
- Banerjee J, Canamar CP, Voyageur C, Tangpraphaphorn S, Lemus A, Jr CC, et al. Mortality and readmission rates among patients with COVID-19 after discharge from acute care setting with supplemental oxygen. JAMA. 2021;4(4):e213990. https://doi:10.1001/jamanetworkopen.2021.3990 [Crossref]
- Akoglu H. User's guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91-3. doi: 10.1016/j.tjem.2018.08.001 [Crossref]
- Ayres M, Ayres JR, Ayres DL,
Santos AS. BioEstat 5.3:
Statistical applications in the areas of Biological and Medical Sciences. Belém: Mamirauá Civil Society
Publishing House; 2011. p. 364.
- Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;58(3):2004015. doi: 10.1183/13993003.04015-2020 [Crossref]
- Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6 [Crossref]
- Gobbi M, Bezzoli E, Ismelli F, Trotti G, Cortellezzi S, Meneguzzo F, et al. Skeletal muscle mass, sarcopenia and rehabilitation outcomes in post-acute COVID-19 patients. J Clin Med. 2021;10(23):5623. doi: 10.3390/jcm10235623 [Crossref]
- Park CH, Yi Y, Do JG, Lee YT, Yoon KJ. Relationship between skeletal muscle mass and lung function in Korean adults without clinically apparent lung disease. Medicine (Baltimore). 2018;97(37):e12281. doi: 10.1097%2FMD.0000000000012281 [Crossref]
- Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3-19. doi: 10.1002/jcsm.12238 [Crossref]
- Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14(1):85-99. doi: 10.1177/1479972316679664 [Crossref]
- Alonso AC, Silva-Santos PR, Quintana MSL, Silva VC, Brech GC, Barbosa LG, et al. Physical and pulmonary capacities of individuals with severe coronavirus disease after hospital discharge: A preliminary cross-sectional study based on cluster analysis. Clinics. 2021;76:e3540. doi: 10.6061/clinics/2021/e3540 [Crossref]
- Moonen HPFX, Zanten FJLV, Driessen L, Smet V, Slingerland-Boot R, Mensink M, et al. Association of bioelectric impedance analysis body composition and disease severity in COVID-19 hospital ward and ICU patients: The BIAC-19 study. Clin Nutr. 2021;40(4):2328-36. doi: 10.1016%2Fj.clnu.2020.10.023 [Crossref]
- Kellnar A, Hoppe JM, Brunner S, Stremmel C. Hospitalization for COVID-19 is associated with significant changes in body composition. Clin Nutr. ESPEN 2021;45:499-502. doi: 10.1016/j.clnesp.2021.07.033 [Crossref]
