Fisioter Bras.
2023;24(3):371-84
REVIEW
Effects of cycle ergometer use in the postoperative
period on functional capacity and hospitalization time in adults undergoing
cardiac surgery: systematic review protocol
Efeitos
do cicloergômetro no pós-operatório sobre a
capacidade funcional e o tempo de hospitalização em adultos submetidos a
cirurgia cardíaca: protocolo de revisão sistemática
João
Paulo Rodrigues Pacheco1, Eduardo Pinheiro Leão1, Ana
Carolina Pereira Nunes Pinto1, Adilson Mendes1, Larissa
de Magalhães Doebeli Matias1, Ioan Cosmin Boca2,
Juliana Ribeiro Fonseca Franco de Macedo3, Adriana Claudia Lunardi4,5,
Elinaldo da Conceição dos Santos1
1Universidade Federal do Amapá, Macapá, Brazil
2University of
Oradea, Oradea, Romania
3Université Catholique
de Louvain, Belgium
4Universidade Cidade de São Paulo, São
Paulo, Brazil
5Universidade de São Paulo, São Paulo, Brazil
Received: December 14,
2022; Accepted: April 25,
2023.
Correspondence: Elinaldo da Conceição dos Santos, drelinaldo@gmail.com
How to cite
Pacheco JPR, Leão EP, Pinto ACPN, Mendes A, Matias LMD, Boca IC, Macedo JRFF, Lunardi AC, Santos EC. Effects of cycle ergometer use in the postoperative period on functional capacity and hospitalization time in adults undergoing cardiac surgery: systematic review protocol. Fisiotere Bras. 2023.24(3):371-84. doi: 10.33233/fb.v24i3.5362
Abstract
Introduction: Among patients
undergoing cardiac surgery, some pulmonary and cardiac complications can be
observed, which can increase the length of hospital stay. The cycle ergometer
is used to try to improve this clinical scenario. However, some inconsistencies
can be observed in the literature. Objective: To synthesize evidence on
the effects of cycle ergometer use in the in-hospital postoperative period in
relation to control therapy without cycle ergometer use on functional capacity
and length of hospital stay in adults undergoing cardiac surgery. Methods:
Systematic review of randomized clinical trials with patients undergoing
cardiac surgery, to evaluate the effects of cycle ergometer use compared to
control without cycle ergometer use. Primary outcomes: functional capacity and
length of hospital stay. Searches: Medline, EMBASE, PEDro,
Pubmed, Allied and AMED, Cinahl,
Lilacs, Scielo, Scopus and Cochrane Central,
ClinicalTrials.gov, ReBEC, and the references of
included studies. Study selection will be conducted by three authors. The risk
of bias will be assessed by two independent authors using the Cochrane Risk of
Bias 2 (RoB 2) tool and conflicts will be resolved
through consensus (in the absence of consensus, a third author will make the
decision). The inverse variance method and random effects model will be
considered in the meta-analysis. Continuous variables will be analyzed by
weighted mean difference and dichotomous variables by relative risk (RR). We
will use I2 statistics to estimate the amount of heterogeneity between studies.
Discussion: Different cardiac surgeries are performed all over the
world, and have been widely investigated. However, some gaps and controversies
can be observed. Therefore, a systematic review is essential to clarify existing
gaps.
Keywords: aerobic exercise; cardiac
surgery; postoperative period.
Resumo
Introdução: Entre os pacientes submetidos à
cirurgia cardíaca, pode-se observar algumas complicações pulmonares e
cardíacas, o que pode aumentar o tempo de hospitalização. O cicloergômetro
é usado para tentar melhorar esse cenário clínico. Contudo, algumas
inconsistências podem ser observadas na literatura. Objetivo: Sintetizar
as evidências sobre os efeitos do cicloergômetro no
pós-operatório intra-hospitalar em relação a terapia de controle sem cicloergômetro na capacidade funcional e tempo de
hospitalização em adultos submetidos à cirurgia cardíaca. Métodos:
Revisão sistemática de ensaios clínicos randomizados com pacientes submetidos a
cirurgias cardíacas avaliando os efeitos do ciclo ergômetro comparado a
controle sem ciclo ergômetro. Desfechos primários: capacidade funcional e tempo
de hospitalização. Buscas: Medline, EMBASE, PEDro, Pubmed, Allied e AMED, Cinahl, Lilacs, Scielo, Scopus e Cochrane Central, ClinicalTrials.gov, ReBEC e nas referências dos estudos incluídos. A seleção do
estudo será conduzida por três autores. O risco de viés será avaliado por dois
autores independentes por meio da ferramenta Cochrane Risk of
Bias 2 (RoB 2) e os conflitos serão sanados mediante
consenso (na falta de consenso, um terceiro autor tomará a decisão). O método
de variância inversa e modelo de efeitos aleatórios serão considerados na metanálise. As variáveis contínuas serão analisadas pela
diferença de média ponderada e as dicotômicas através do risco relativo (RR).
Usaremos estatística de I2 para estimar a quantidade de heterogeneidade entre
os estudos. Discussão: Diferentes cirurgias cardíacas são realizadas em
todo o mundo, sendo amplamente investigadas. No entanto, algumas lacunas e
controvérsias são observadas. Portanto, uma revisão sistemática é essencial
para esclarecer as lacunas existentes.
Palavras-chave: exercício aeróbico; cirurgia cardíaca;
período pós-operatório.
Introduction
Cardiac surgeries are often highly complex
surgical procedures and are therefore concentrated in well-developed urban
areas and in low- and middle-income countries. In general, there are three
types of cardiac surgery: corrective, reconstructive, and substitutive.
Approximately 2.5 million people with cardiovascular disease require heart
surgery [1]. This demand for heart surgery appears to increase year on year,
for example, in 2020 in Germany there was a 2.1% increase in heart transplants
compared to 2019 [2]. In Brazil, in the last three years, 874 heart
transplants, 5,222 myocardial revascularizations using cardiopulmonary bypass,
and 6,104 valve repairs and/or multiple valve replacement were performed [3].
Among patients who undergo cardiac
surgery, 66.6% develop postoperative complications, 47.3% require blood
products, 32% have atrial fibrillation complications, 0.9% have cardiac arrest,
and 2.7% present pneumonia [4]. Postoperative hospital stays of 21 days in the
intensive care unit and 24.6 days in the ward were reported [5].
In addition, loss of functional
capacity, person's ability to exercise self-care and live independently [6],
has been observed after cardiac surgery, being greatest on the seventh
postoperative day [7]. When the patient presents associated complications, this
functional loss is even greater, with reductions in muscle strength of the
lower limbs and handgrip strength, which may further increase the
hospitalization time [8].
In an attempt to reduce these
complications and hospitalization time, as well as to improve the functional
capacity of the patient, some treatment strategies are used in the
postoperative period [9,10], for example, the cycle ergometer, which represents
an alternative treatment used in upper and lower limbs, that appears to make
therapy more attractive and engaging for the patient undergoing cardiac surgery
[11]. However, some inconsistencies can be observed in the literature,
regarding the relationship between the use of a cycle ergometer and physical
activity, safety [10], cardiac autonomic modulation, length of hospital stay,
and functionality [12,13,14]. These inconsistencies show the need for a systematic
review to clarify these doubts. At least two clinical trials evaluating the
effects of the cycle ergometer in the postoperative period of cardiac surgery
have already been published, making this systematic review feasible [15,16].
Therefore, it is necessary to
address these questions about the use of the cycle ergometer in the
postoperative period of cardiac surgery, in order to conduct the treatment
appropriately, with safety, and above all, knowing what to expect when this
therapy is used in clinical practice while the patient is hospitalized. After
conducting a search of PROSPERO, Cochrane Library, Pubmed
and JBI Evidence Synthesis, ongoing or published reviews on the review topic
were found. Therefore, this review aims to answer the following research
question: - Is the cycle ergometer more effective than control therapy without
a cycle ergometer in the in-hospital postoperative period (phase I of
cardiovascular rehabilitation) of cardiac surgery in adults on functional
capacity, hospitalization time, cardiac and pulmonary complications, heart
rate, blood pressure, perception of exertion, and adverse events?
Methods
Design
We report this protocol in line
with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Protocols (PRISMA-P) [17]. This review protocol was previously registered in
the International Prospective Register of Systematic Reviews (PROSPERO) under
CRD42022378883.
Eligibility criteria
The eligibility criteria were
prepared according to the PICO (Population, Intervention, Comparison, end
Outcome) mnemonic.
Types of studies, language, and
year of publication: Only randomized controlled trials published in any
language and in any year will be included in this review.
Types of participants: Included
studies are required to have been conducted with adult participants (at least
18 years of age) undergoing cardiac surgery.
Types of intervention: Clinical
trials that evaluated the effect of the cycle ergometer in the in-hospital
postoperative period (phase I of cardiovascular rehabilitation) of cardiac
surgery will be included. The cycle ergometer could be used alone or in
combination with other techniques, for at least 2 days.
Types of comparatives: Comparative
therapy was required to be usual care Control without a cycle ergometer
(Breathing exercises, non-invasive ventilation, and conventional physical
therapy.
Investigated outcome: Having assessed
at least one of the outcomes investigated by this systematic review
Primary outcomes
Functional
capacity: Functional capacity will be recorded regardless of
the method or scale used to measure this outcome after cardiac surgery, e.g.,
six-minute walk test [18], Incremental Shuttle Walk Test, one-minute
sit-to-stand test [19], among others.
Hospitalization
time: The total number of days the patient stays in the
hospital will be recorded. In the case of studies reporting intensive care unit
length of stay and ward length of stay separately, the sum of days will be
performed to obtain the total hospitalization time.
Secondary outcomes
Cardiac (e.g. cardiac arrhythmia,
acute myocardial infarction, orthostatic hypotension, and pneumopericardium)
and pulmonary (e.g. acute respiratory failure, pleural effusion, hypoxemia,
pneumonia, and atelectasis) complications: A complication is an unfavorable
result after heart surgery. Complications may adversely affect the outcome of
surgical procedure. The number of events (complications) will be recorded.
Heart rate: The number of heart
beats in each minute after cardiac surgery will be recorded. Patients
undergoing cycle ergometer training show variability in heart rate and blood
pressure, which is also considered a way to detect autonomic instability in the
postoperative phase, thus allowing to predict or prompt-diagnose postoperative
complications [20]. All forms of heart rate monitoring will be accepted.
Systolic, diastolic, and mean blood
pressure: Systolic blood pressure, diastolic blood pressure, and mean blood
pressure measured using digital or analogue devices after cardiac surgery will
be recorded.
Perception of effort: A rational
notion of how arduous and exhausting it is to perform a given physical task
[21] will be recorded. Studies that measured the perception of exertion by
measuring instruments such as the modified Borg scale, but not limited to it,
will be considered.
Adverse events: Adverse
healthcare-related events are incidents that occur during medical care and harm
a patient, producing an injury, suffering, disability, or death [22]. Adverse
events related to the use of the cycle ergometer, such as muscle pain, fatigue,
nausea, among others, will be considered and recorded.
Information sources
The following databases will be
searched: Medline (Through the EBSCOhost Research Platform), EMBASE, PEDro, Pubmed, AMED, Cinahl, Lilacs, Scielo, Scopus,
and Central. The search will also be conducted in two clinical trial registry
bases: ClinicalTrials.gov and ensaiosclinicos.gov.br. Finally, we will perform
a search in the references of the studies included through the Snowballing
technique and search for citations of studies selected for the synthesis
through the Forward Citation Searching technique. Related descriptors and
synonyms will be used, to adapt the search to the conditions of each source.
Search strategy
Terms related to the problem of
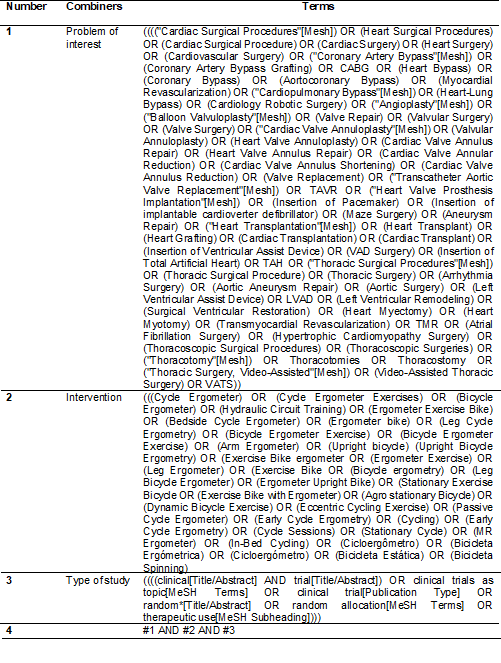
interest and the therapeutic technique will be used. The terms are described in
Table I. The search strategy below will be used in Medline via Pubmed and will be adapted to the specifications of each database.
Table I - Systematic review search
strategy
Selection of studies
Three authors will independently
select studies for inclusion in this systematic review (AM, ICB, and ECS). Two
authors will extract possible studies identified based on the eligibility
criteria (ICB and ECS). The authors will read the studies in the following
order: titles, abstracts, and, if necessary, the texts will be read in full to
decide on the study’s eligibility for inclusion. In the case of inconsistency
between the two authors about the inclusion of the study in this review, an
attempt will be made to reach an agreement between the two authors, and if the
inconsistency persists, the inclusion of the study will be resolved by the
third author (AM). Studies that do not meet the criteria will be excluded. In
addition, studies in more than one database (duplicates), and studies with a
smaller sample size with the same participants, the same outcome measures, and
the same follow-up time for evaluations (duplicate reporting), will be
excluded. Rayyan software [23] will be used to streamline the screening and
selection of studies. The flowchart that will be followed to report the
selection process of this systematic review is shown in Figure 1.
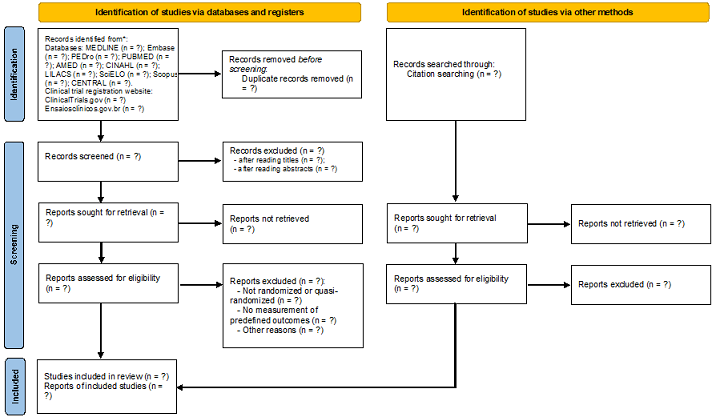
Figure 1 - Flowchart of the systematic
review
Extraction of data from studies
Using a previously standardized
method, two independent authors (JPRP and EPC) will extract data from the
selected studies. A third author (ACNP) will monitor possible discrepancies in
relation to the extracted data. If there is a discrepancy, this third author
will make the final decision. A spreadsheet in the Excel application
(Elaborated by the authors) will be used to record the extracted data, such as
general characteristics of the studies (Authors, year and language of
publication, and study design), patients involved (Sample size, sex, age, and
clinical characteristics), surgical procedures included (Type of surgery,
duration of surgery and use or not of extracorporeal membrane oxygenation), and
outcomes of interest (Mean, standard deviation, median, the smallest value
(Minimum), the largest value (Maximum), confidence interval, estimated
population standard deviation, p value, and standard error will be extracted)
in this systematic review.
Methodological robustness of included clinical trials
and certainty of evidence
Two independent authors (JRFFM and
LMDM) will assess the risk of bias. A third author (ACL) will monitor possible
inconsistencies in the assessment. If there is inconsistency, an agreement on
the decision in the evaluation will be prioritized between the two authors
(JRFFM and LMDM). If there is no agreement, the final decision will be made by
the third author (ACL).
The risk of bias will be assessed
according to the RoB 2 tool, which consists of five
levels. Within each level, RoB
2 users answer one or more signaling questions. Assessment responses are rated
as low risk of bias, some concerns, or high risk of bias. Assessments at each
level result in an overall risk of bias assessment for the judged outcome,
which allows RoB 2 users to stratify meta-analyses by
risk of bias [24,25]. Briefly, details of the randomization method with
sequence generation, allocation concealment, degree of blinding, inclusion and
exclusion criteria, study dropouts or withdrawals, intent to treat, and
detailed statistical analysis will be examined.
Unexplained dropouts or an uneven
number of dropouts across treatment groups will be considered a potential risk
of bias. Likewise, the lack of important information, for example missing data,
statistical methods, etc., will also be considered a potential risk of bias.
Studies with low methodological robustness will not be excluded from the
review. We will assess the quality of evidence using GRADE (Grading of
Recommendations, Assessment, Development and Evaluations), using GRADEpro GDT [26]. GRADE is an accessible and comprehensive
approach that guides assessments on the certainty of evidence. The GRADE
assessment is based on the overall risk of bias, consistency of results,
objectivity of evidence, publication bias, and accuracy of each outcome [27].
Evaluation of the quality of description of clinical
trials
The reporting quality of the
included studies will be assessed using the TIDieR
(Template for intervention description and replication) checklist. The TIDieR was developed with the aim of improving the
reporting of interventions in randomized controlled trials [28,29]. The
checklist contains 12 items, including: intervention name, rationale,
intervention materials, details of intervention providers, mode of intervention
delivery, intervention delivery location and infrastructure, details of the
number, duration, intensity and dose of interventions, intervention sessions,
details of adaptations of any intervention, any modifications throughout the
study, assessment of fidelity, monitoring and level reached [28,29]. We will
perform the sum of each item for the control and intervention groups, and each
item will be evaluated on a three-point Likert scale according to the following
categorizations, with their respective points: not reported (0), partially
reported (1) and adequately reported (2). Thus, summary scores range from 0
(bad report) to 24 (good report) points [28].
Meta-analysis and heterogeneity
The meta-analysis will be performed
using the inverse variance method and the random effects model in RevMan 5 [30]. Continuous variables will be analyzed by the
weighted mean difference with 95% CI. Dichotomous variables will be analyzed
through the RR with 95% CI.
When at least two studies are
sufficiently homogeneous in terms of participants, interventions, and outcome
measures, the results will be pooled in a meta-analysis. Separate meta-analyses
will be performed for studies evaluating short-term (up to 2 months),
medium-term (2 months to 6 months), and long-term (more than 6 months)
outcomes. If a study has more than one measure, for example, in the short term
(e.g. if evaluated in the second week and in the fourth week), we will consider
the latest evaluation.
In case of selection of studies
with insufficient data, the study authors will be contacted to request access
to the missing data. If, for the same outcome, there are at least 10 studies,
the publication bias will be evaluated, and for studies with a small sample
size or in situations when there is doubt in the definition of this bias, we
will use the Egger's test.
We will use the Higgins and
Thompson inconsistency test (I2) to estimate the amount of heterogeneity
between studies in each meta-analysis. I2 values range from 0 to 100%. Values
from 0% to 40% may not be important, values from 30 to 60% may represent
moderate heterogeneity, values from 50% to 90% may represent substantial
heterogeneity, and values from 75% to 100% considerable heterogeneity between
studies [31]. In case of considerable heterogeneity, we will investigate
possible causes by performing subgroup/sensitivity analyses. We will consider
the following subgroups when investigating their effect on heterogeneity: sex,
type of surgery, use of cardiopulmonary bypass, intervention details such as
use of different types of devices (cycle ergometer for upper and lower limbs),
frequency, duration, and start time of the intervention. We will consider the
following information for sensitivity analysis: no blinding or inadequate
blinding of outcome assessors, inadequate randomization methods, and large
numbers (> 20%) of patients lost to follow-up.
Discussion
Different heart surgeries are performed worldwide and
are often complex, for example, coronary, valve, aortic, and heart failure
surgery and therefore, in the last decade, they have been widely investigated
[32]. Regardless of the cause that leads to the need for cardiac surgery, the
physical therapy treatment offered in the postoperative period should be
properly managed to prevent and treat complications and reduce hospitalization
time.
With this objective, the cycle
ergometer is a device that has been used in the postoperative period of cardiac
surgery in clinical practice, in different hospitals, and in different phases
of cardiovascular rehabilitation, including phase I. In addition, some clinical
trials have investigated its effects, showing conflicting results [11,33].
Therefore, a systematic review with a methodologically well-constructed
protocol is essential to clarify existing knowledge gaps around the use of a
cycle ergometer in the postoperative period of cardiac surgery.
Conflicts of interest
The authors declare no conflict of
interest.
Funding
None.
Author contributions
Conception and design
of the study: Pacheco JPR, Leão
EP, Pinto APNP, Matias LMD, Santos EC; Manuscript writing: Pinto APNP,
Lunardi AC, Santos EC; Critical review of the manuscript for important
intellectual content: Mendes A, Pinto APNP, Boca IC, Macedo JRFF, Lunardi
AC, Santos EC
References
- Vervoort D, Meuris B, Meyns B, Verbrugghe P. Global
cardiac surgery: Access to cardiac surgical care around the world. J Thorac Cardiovasc Surg. 2020;159(3):987-996.e6. doi: 10.1016/j.jtcvs.2019.04.039 [Crossref]
- Beckmann A, Meyer R, Lewandowski J, Markewitz A, Gummert J. German
Heart Surgery Report 2020: The Annual Updated Registry of the German Society
for Thoracic and Cardiovascular Surgery. Thorac
Cardiovasc Surg. 2021;69(4):294-307. doi: 10.1055/s-0041-1730374 [Crossref]
- DATASUS tabnet [Internet].
[cited 2022 Sept 10]. Available from:
http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sih/cnv/qiuf.def
- Pahwa S, Bernabei A, Schaff H, Stulak J, Greason K, Pochettino A, et al. Impact of postoperative complications after cardiac surgery on long-term survival. J Card Surg. 2021;36(6):2045-52. doi: 10.1111/jocs.15471 [Crossref]
- Almashrafi A, Alsabti H, Mukaddirov M, Balan B, Aylin P. Factors associated with prolonged length of stay following cardiac surgery in a major referral hospital in Oman: a retrospective observational study. BMJ Open. 2016;6(6):e010764. doi: 10.1136/bmjopen-2015-010764 [Crossref]
- Pinto AH, Lange C, Pastore CA, Llano PMP, Castro DP, Santos F. Functional capacity to perform activities of daily living among older persons living in rural areas registered in the Family Health Strategy. Ciênc Saúde Coletiva. 2016;21(11):3545-55. doi: 10.1590/1413-812320152111.22182015 [Crossref]
- Borges JBC, Ferreira DLMP, Carvalho SMR, Martins AS, Andrade RR, Silva MAM. Pain intensity and postoperative functional assessment after heart surgery. Braz J Cardiovasc Surg. 2006;21(4):393-402. doi: 10.1590/S0102-76382006000400009 [Crossref]
- Sumin AN, Oleinik PA, Bezdenezhnykh AV, Bezdenezhnykh NA. Factors determining the functional state of cardiac surgery patients with complicated postoperative period. Int J Environ Res Public Health. 2022;19(7):4329. doi: 10.3390/ijerph19074329 [Crossref]
- Santos TD, Pereira SN, Portela LOC, Cardoso DM, Lago PD, Guarda NDS, et al. Moderate-to-high intensity inspiratory muscle training improves the effects of combined training on exercise capacity in patients after coronary artery bypass graft surgery: A randomized clinical trial. Int J Cardiol. 2019;279:40-46. doi: 10.1016/j.ijcard.2018.12.013 [Crossref]
- Busch JC, Lillou D, Wittig G, Bartsch P, Willemsen D, Oldridge N, at al. Resistance and balance training improves functional capacity in very old participants attending cardiac rehabilitation after coronary bypass surgery. J Am Geriatr Soc. 2012;60(12):2270-6. doi: 10.1111/jgs.12030 [Crossref]
- Lordello GGG, Gama GGG, Rosier GL, Viana PADC, Correia LC, Fonteles Ritt LEF. Effects of cycle ergometer use in early mobilization following cardiac surgery: a randomized controlled trial. Clin Rehabil. 2020;34(4):450-59. doi: 10.1177/0269215520901763 [Crossref]
- Ribeiro BC, Poça JJG, Rocha AMC, Cunha CNS, Cunha KC, Falcão LFM, et al. Different physiotherapy protocols after coronary artery bypass graft surgery: A randomized controlled trial. Physiother Res Int. 2021;26(1):e1882. doi: 10.1002/pri.1882 [Crossref]
- Haennel RG,
Quinney HA, Kappagoda CT. Effects of hydraulic
circuit training following coronary artery bypass surgery. Med Sci Sports Exerc [Internet]. 1991 [cited 2022 Sept 10];23(2):158-65.
Available from: https://pubmed.ncbi.nlm.nih.gov/2017011
- Forestieri P, Guizilini S, Peres M, Bublitz C, Bolzan DW, Rocco IS, et al. A cycle ergometer exercise program improves exercise capacity and inspiratory muscle function in hospitalized patients awaiting heart transplantation: a pilot study. Braz J Cardiovasc Surg. 2016;31(5):389-95. doi: 10.5935/1678-9741.20160078 [Crossref]
- Trevisan MD, Lopes DGC, Mello RGB, Macagnan FE, Kessler A. Alternative physical therapy protocol using a cycle ergometer during hospital rehabilitation of coronary artery bypass grafting: a clinical trial. Braz J Cardiovasc Surg. 2015;30(6):615-9. doi: 10.5935/1678-9741.20150085 [Crossref]
- Foestieri P, Guizilini S, Peres M, Bublitz C, Bolzan DW, Rocco IS, et al. A cycle ergometer exercise program improves exercise capacity and inspiratory muscle function in hospitalized patients awaiting heart transplantation: a pilot study. Braz J Cardiovasc Surg. 2016;31(5):389-95. doi: 10.5935/1678-9741.20160078 [Crossref]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4:1. doi: 10.1186/2046-4053-4-1 [Crossref]
- Baptista VC, Palhares LC, Oliveira PPM, Filho LMS, Vilarinho KAS, Severino ESBO, et al. Six-minute walk test as a tool for assessing the quality of life in patients undergoing coronary artery bypass grafting surgery. Rev Bras Cir Cardiovasc. 2012;27(2):231-9. doi: 10.5935/1678-9741.20120039 [Crossref]
- Winkelmann ER, Silva LG, Silva MMD, Windmoller P, Silva AB. Cardiopulmonary responses of the sit-to-stand test in individuals pre and post-heart surgery: cross section. Journals Bahiana School of Medicine and Public Health. 2021;11(4):730-37. doi: 10.17267/2238-2704rpf.v11i4.4127 [Crossref]
- Nenna A, Lusini M, Spadaccio C, Nappi F, Greco SM, Barbato R, et al. Heart rate variability: a new tool to predict complications in adult cardiac surgery. J Geriatr Cardiol. 2017;14(11):662-68. doi: 10.11909/j.issn.1671-5411.2017.11.005 [Crossref]
- Pageaux B. Perception of effort in Exercise Science: Definition, measurement and perspectives. Eur J Sport Sci. 2016;16(8):885-94. doi: 10.1080/17461391.2016.1188992 [Crossref]
- World Health Organization & WHO Patient Safety.
Conceptual framework for the international classification for patient safety
version 1.1: final technical report January 2009. World Health Organization.
https://apps.who.int/iris/handle/10665/70882
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan – a web and mobile app for systematic review. Systematic Reviews. 2016;5:210. doi: 10.1186/s13643-016-0384-4 [Crossref]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe
NS, Boutron I, et al. RoB 2: a
revised tool for assessing risk of bias in randomised
trials. BMJ. 2019;28;366:l4898. doi: 10.1136/bmj.l4898 [Crossref]
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page
M, et al. Cochrane Handbook for Systematic Reviews of
Interventions version 6.0. Cochrane. 2019.
- GRADEpro GDT
2015. Grade’s software for summary of findings tables, health technology
assessment and guidelines. https://gradepro.org/
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490 [Crossref]
- Yamato TP, Maher CG, Saragiotto BT, Catley MJ, Moseley AM. Rasch analysis suggested that items from the template for intervention description and replication (TIDieR) checklist can be summed to create a score. J Clin Epidemiol. 2018;101:28-34. doi: 10.1016/j.jclinepi.2018.05.014 [Crossref]
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [Crossref]
- Review Manager 5 (RevMan 5)
[Computer program]. Version 5.3. Copenhagen: Nordic Cochrane Centre,
Cochrane; 2014.
- Higgins J, Green S (e). Cochrane Handbook for
Systematic Reviews of Interventions Version 5.1.0, The Cochrane Collaboration; 2011.
- DoenstT, Bargenda S, Kirov H, Moschovas A, Tkebuchava S, Safarov R, et al. Cardiac Surgery 2019 Reviewed. Thorac Cardiovasc Surg. 2020;68(5):363-76. doi: 10.1055/s-0040-1713648 [Crossref]
- Borges DL, Silva MG, Silva LN, Fortes JV, Costa ET, Assunção RP, et al. Effects of aerobic exercise applied early after coronary artery bypass grafting on pulmonary function, respiratory muscle strength, and functional capacity: a randomized controlled trial. J Phys Act Health. 2016;13(9):946-51. doi: 10.1123/jpah.2015-0614 [Crossref]
