Fisioter Bras. 2023;24(6):979-96
REVISÃO
Comparison between training protocols with blood flow
restriction in clinical musculoskeletal rehabilitation: protocol of a
systematic review
Comparação
entre os protocolos de treinamento com restrição do fluxo sanguíneo na
reabilitação clínica musculoesquelética: protocolo de uma revisão sistemática
Roger
Andrey Carvalho Jardim, Ingrid Nazaré Lourinho Alves, Areolino
Pena Matos, Ana Carolina Pereira Nunes Pinto, Natália Camargo Rodrigues
Iosimuta
Universidade
Federal do Amapá, Macapá, AP, Brazil
Received July 7, 2023; Accepted:
August 15, 2023.
Correspondence: Natália Camargo Rodrigues Iosimuta, naticrod@unifap.br
How to cite
Jardim RAC, Alves INL, Matos AP, Pinto
ACPN, Iosimuta NCR. Comparison between
training protocols with blood flow restriction in clinical musculoskeletal rehabilitation:
protocol of a systematic review. Fisioter Bras. 2023;24(6): 10.33233/fb.v24i6.5502
Abstract
Introduction: The training
with blood flow restriction (RFS) is increasingly used in the clinical
rehabilitation of musculoskeletal disorders, but there is still much
uncertainty about which parameters is ideal to use regarding the restriction
pressure, combination with other therapies and which groups of patients can
benefit most from the technique. Objective: To compare the effectiveness
and safety of different methodologies for the use of RFS in the rehabilitation
of musculoskeletal disorders. Methods: A systematic review will be
performed through independent searches by two reviewers of studies indexed to
Medline, SPORTDiscus, Central, Lilacs, CINAHL, Embase, J-STAGE and PEDro; in
addition to sources of gray literature and in the bibliographic references of
the studies included in the review. The study protocol was registered on the
Prospero Platform (CRD422021233488). Randomized parallel-group clinical trials
that tested the efficacy or safety of RFS training in individuals aged 18 years
or older for at least three weeks will be considered. Details will be extracted
on the application of RFS and outcomes of pain, muscle strength and adverse
events, considered as primary outcomes; in addition to functional capacity,
discomfort, overall physical capacity and quality of life, composing the
secondary outcomes. The risk of bias of the studies will be assessed using the PEDro scale and any disagreement during the process of
selection, extraction and evaluation of the risk of bias will be decided by a
third reviewer. Expected results: Expose the information available in
the literature and provide clear guidance to clinicians and researchers on the
prescriptions and recommendations of this technique.
Keywords: musculoskeletal rehabilitation;
restriction of blood flow.
Resumo
Introdução: O treino com restrição de fluxo
sanguíneo (RFS) é cada vez mais utilizado na reabilitação clínica das
disfunções músculo-esqueléticas, mas ainda existe
muita incerteza sobre que parâmetros ideais para serem utilizados quanto à
pressão de restrição, combinação com outras terapias e quais os grupos de
pacientes que mais podem se beneficiar da técnica. Objetivo: Comparar a
eficácia e a segurança de diferentes metodologias de utilização do RFS na
reabilitação de disfunções musculoesqueléticas. Métodos: Será realizada
uma revisão sistemática por meio de buscas independentes por meio de dois
revisores de estudos indexados ao Medline, SPORTDiscus,
Central, Lilacs, Cinahl,
Embase, J-STAGE e PEDro; além de fontes da literatura
cinza e nas referências bibliográficas dos estudos incluídos na revisão. O
protocolo do estudo foi registrado na Plataforma PROSPERO (CRD422021233488).
Serão considerados ensaios clínicos randomizados de grupos paralelos que
testaram a eficácia ou segurança do treinamento RFS com indivíduos com 18 anos
ou mais por pelo menos três semanas. Serão extraídos detalhes sobre a alicação do RFS e desfechos de dor, força muscular e
eventos adversos, considerados como desfechos primários; além da capacidade
funcional, desconforto, capacidade física geral e qualidade de vida, compondo
os desfechos secundários. O risco de viés dos estudos será avaliado por meio da
escala PEDro
e qualquer discordância durante o
processo de seleção, extração e
avaliação do risco de viés será decidida
por um
terceiro revisor. Resultados esperados: Expor as informações disponíveis
na literatura e fornecer orientações claras aos clínicos e pesquisadores sobre
as prescrições e recomendações desta técnica.
Palavras-chave: reabilitação musculoesquelética; restrição
do fluxo sanguíneo.
Introduction
The regular practice of physical
exercise is an indispensable resource for health promotion at the individual
and collective level, being a tool for prevention and rehabilitation in various
health conditions [1]. For the benefits of physical exercise to be optimized,
it must be practiced under minimum parameters of frequency and intensity [2].
The World Health Organization (WHO) recommends that individuals with chronic
diseases practice weekly at least 75 minutes or 150 minutes of moderate or
intense aerobic activity, respectively [3]. In addition, the incorporation of
muscle strengthening exercises with intensity between 60-70% of one maximum
repetition (1RM) is recommended, as it offers additional benefits [3,4].
However, for some groups of
patients, the use of conventional physical exercises presents limitations,
because the minimum recommended training parameters are above the physical
capacities of the patients, and alternatives are necessary for the benefits of
physical activity to be enjoyed by these populations [5]. In this sense,
training with blood flow restriction (BFR) has been a viable solution because
it demands a high physical intensity even with low loads and less joint stress,
which is the training condition desired for the clinical rehabilitation of
patients with musculoskeletal disorders (MSD) [6,7].
The BFR training consists of
placing a partially inflated cuff or tourniquet in the proximal region of a
muscle group in which blood flow is desired, with the objective of evoking in a
shorter time local and systemic physiological responses
with low loads similar to those offered by moderate and high intensity training
for a prolonged time [8].
In previous systematic reviews, BFR
has been shown to be effective in the clinical rehabilitation of patients with
MSD in terms of muscle strength, pain and muscle volume [9-11]. However, the
high heterogeneity of BFR use in the studies raised prevents a concise
recommendation on appropriate parameters that achieve the desirable therapeutic
effects with the technique [12,13].
The most common clinical form of
BFR use is the combination with resistance or aerobic exercises of low
intensity, but it can be combined with high and moderate intensity training, or
even used at rest [8]. In addition, the choice of blood flow restriction
pressure (BFRP) is quite heterogeneous and can be based on formulas,
percentages of occlusion pressure of each individual, diastolic pressure or
absolute values [12].
Systematic reviews on the use of
BFR in the rehabilitation of MSD are conducted focusing on lower limb
pathologies, such as knee osteoarthrosis, patellofemoral pain syndrome and
anterior cruciate ligament injury [14]. In addition, the studies included in
the available reviews use protocols based exclusively on resistance exercises,
excluding interventions that combine with aerobic exercise [7,9-11,13].
Thus, the current available
literature does not provide an overview of the effectiveness of BFR in the
rehabilitation of patients with MSD, in addition to grouping different
methodologies for the use of BFR in the same analyses. Therefore, the aim of
this study is to compare the methods of using BFR in the clinical
rehabilitation of MSD, regardless of the site of the lesion and considering the
characteristics of the therapeutic approaches employed.
Methods
Type of study
This is a protocol of systematic
review of the literature to compare the efficacy and safety of different BFR
training methodologies in patients diagnosed with MSD. All stages of the study
will be conducted in accordance with the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Ethical and local aspects of research
The review protocol was registered
in the International Prospective Register of Systematic Reviews (PROSPERO)
platform (CRD422021233488). This study will be conducted at the Federal
University of Amapá (UNIFAP), in the Post-Graduate
Program in Health Sciences.
Search strategy
Searches will be performed in the
following databases: Medline, SPORTDiscus, Central,
Lilacs, Cinahl, Embase,
J-STAGE and PEDro. In addition to searches in gray
literature in bases such as OpenGrey and ProQuest.
Search terms and keywords will be tailored to each database as appropriate. In
addition, searches will be carried out in the bibliographic references of all
articles included, in order to identify possible sources eligible for review.
There will be no language
restriction, sources that are not in Portuguese, Spanish and English, will be
forwarded to professional translation services. In cases where the absence of
relevant data is identified, authors will be contacted to request additional
information.
Chart I – Search strategies of the
systematic review
Types of studies included
Randomized clinical trials that
tested the efficacy or safety of RFS training compared to a control group for a
minimum of three weeks will be included.
Types of participants
The studies should have as
population of interest adults (≥ 18 years) with MSD, defined by the WHO
(2003) as a set of more than 150 diseases that affect the locomotor system of
individuals, ranging from acute conditions of restricted duration, such as
sprains, fractures and strains, to lifelong conditions that can lead to
limitations and continuous disabilities, such as rheumatoid arthritis,
fibromyalgia and osteoarthrosis.
Primary
outcomes
- Pain: Measured by patient-reported pain assessment
instruments (e.g., visual analog scale, numerical pain scale, or any other
validated instrument).
- Muscle strength: Measured by an objective assessment
(e.g., isokinetic dynamometer, manual, or any other validated instrument).
- Adverse events: Defined by the WHO [3] as an
incident that results in harm to the patient, including lesions of biological
tissues, functional limitations or any deleterious effect resulting from the
intervention.
Secondary outcomes
- Discomfort: Measure of discomfort resulting from the
use of RFS evaluated by validated instruments, such as the visual analog scale.
- Functional capacity: It refers to the ability to
perform tasks and activities that the individual considers necessary or
desirable for his life, and should be measured by instruments validated for the
specific condition of the patient (for example, Lequesne
Functional Questionnaire, Roland Morris Disability Questionnaire, among
others).
- Overall physical capacity: Related to physical
performance evaluated experimentally under conditions planned by validated
instruments (e.g., Sit and Stand Up Test, 6-minute Walk Test, among others).
- Quality of life: According to the WHO (1995) quality
of life is "the individual's perception of his insertion in life, in the
context of the culture and value systems in which he lives and in relation to
his goals, expectations, standards and concerns". It is a broad concept,
affected in a complex way by physical health, psychological, mental, emotional
state, level of independence, social relationships and other life
circumstances. Measures of validated instruments will be considered, such as
SF-36, WHOQOL-100, among others.
Selection of studies
First, the titles and abstracts
will be compared by two authors independently with a checklist of the
pre-specified inclusion and exclusion criteria. The abstracts of the studies
considered eligible, and those for which it is not possible to state whether
the inclusion criteria were met, will be forwarded to the full-text screening
stage.
The full texts of all potentially
eligible articles will then be checked and compared with the inclusion and
exclusion criteria checklist, again, by two reviewers independently, as described
above. If at any stage of the process the two reviewers are not convinced as to
the eligibility of any particular study, or there are discrepancies about the
eligibility judgments, a third reviewer will be consulted and eligibility
decided by consensus. Each step of the study selection process will be
documented in a flowchart, following the PRISMA format and recommendation as
shown in (Figure 1).
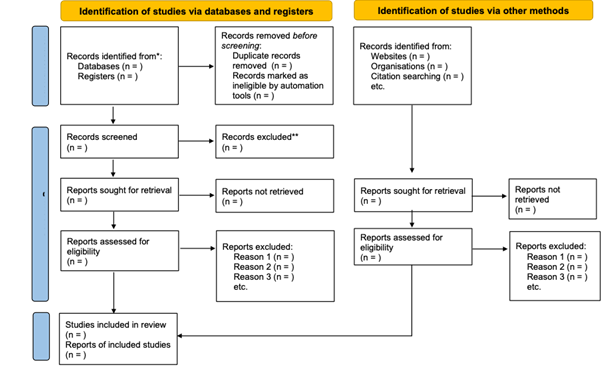
Figure 1 – Systematic review flowchart
Data extraction and management
Data from the included studies will
be extracted using a standardized form in PICO format (population,
intervention, comparison, outcomes, and study design). Specifically, we plan to
collect details about RFS application, study design characteristics, and
information about participants. Age, gender, diagnostic criteria and severity
of disease at baseline of the groups, details on the number of participants
selected, randomized, analyzed, excluded and lost at follow-up.
On the use of the RFS will be
collected information about the combination with other therapies,
characteristics of the equipment used, region of application of the technique,
frequency, time, intensity, interval, methodology for determining the
restriction pressure and strategies to ensure the pressure selected during the
intervention.
In addition, information on the
method of randomization and concealment of allocation, blinding, use of
stratification, incomplete outcome data, selective reporting, use of
intention-to-treat analysis, duration of the study, outcome measures
(continuous and dichotomous), reported time points, funding for the trial, and
notable conflicts of interest of the trial authors.
Data eligible for subgroup analysis
(percentage of blood flow restriction training restriction, training intensity,
and number of joints affected) will also be collected in this phase. The
extracted information will be manually entered into the Review Manager (RevMan) for synthesis and analysis.
Assessment of the risk of bias
The PEDro
scale will be used to assess the risk of bias in the included studies. If
available, the scores provided in the database platform (pedro.org.au) will be
used. In unavailable cases, the PEDro scale will be
applied by two independent authors. For discordant scores, a third reviewer
will decide the outcome.
Trials with a score ≥ 6 will
be considered as 'low risk' of bias; on the other hand, trials with a score
< 6 will be considered as 'high risk' of bias [15].
Strategy for data synthesis
The statistical, methodological and
clinical heterogeneity of the studies will be evaluated. The last two will be
based on the methodology of application of the RFS and the characteristics of
the population. Statistical heterogeneity will be assessed using the I²
statistic, as defined by Higgins et al. in the Cochrane Handbook [16],
being categorized as follows:
1. 0% to 40%: may not be important;
2. 30% to 60%: may represent moderate heterogeneity;
3. 50% to 90%: may represent substantial
heterogeneity;
4. 75% to 100%: substantial heterogeneity.
In cases of substantial statistical
heterogeneity, sensitivity analysis will be performed in order to detect
studies that considerably increase the heterogeneity of the analysis. In
addition, sensitivity analyses are planned after the exclusion of trials
identified as at high risk of bias, with the aim of exploring the impact of
risk classification of bias on the effects of interventions.
The results will be grouped and
represented in forest plots only if the studies are sufficiently homogeneous,
which will be analyzed through a pooled quantitative synthesis using a random
effects model [16]. If it is not possible or appropriate to combine the results
due to heterogeneity, the results will be presented narratively. If applicable,
subgroup analyses will be conducted for the percentage of RFS (≤ 50% or ≥
70%), training intensity (low-moderate or high) and for the number of joints
affected (monoarticular or multiarticular).
Two reviewers will independently
assess the quality of the evidence using the Grading of Recommendations
Assessment, Development and Evaluation (GRADE), and disagreements will be
resolved by a third reviewer. Finally, publication bias will be through the
evaluation of funnel plots when 10 or more studies are included in a
meta-analysis.
Expected results
This study aims to conduct a
systematic review to investigate the effectiveness of RFS in the rehabilitation
of patients with MSD. We hope that the results will update the information
available in the literature and provide clear guidance to clinicians and
researchers on the prescriptions and recommendations of this technique, aiming
to achieve the desirable therapeutic effects through blood flow restriction.
However, it is important to highlight that there are common limitations in
systematic review studies, such as the availability and quality of the included
studies, the heterogeneity of the results and possible publication biases.
These limitations should be considered when interpreting the results and
applying the conclusions in clinical practice.
Conflict of interests
The authors have no conflicts of
interest to declare.
Funding
Study not funded.
Author contributions
Conception review: Jardim RAC, Iosimuta NCR, Pinto ACPN and
Matos AP; Identification of studies and extraction of data: Jardim RAC
and Alves INL; Statistical analysis and data interpretation: Jardim RAC,
Iosimuta NCR; Manuscript writing: Jardim RAC,
Alves INL, Iosimuta NCR. All the authors
critically revised the manuscript.
References
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton
JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA - J
Am Med Assoc. 2018;320(19). doi:
10.1001/jama.2018.14854
- O’Donovan G, Blazevich AJ,
Boreham C, Cooper AR, Crank H, Ekelund U, et al. The
ABC of physical activity for health: A consensus statement from the British
association of sport and exercise sciences. J Sports Sci. 2010;28(6). doi: 10.1080/02640411003671212
- Bull FC, Al-Ansari SS, Biddle S, Borodulin
K, Buman MP, Cardon G, et al. World Health
Organization 2020 guidelines on physical activity and sedentary behaviour. Vol. 54, Br J Sports Med. 2020. doi:
10.1136/bjsports-2020-102955
- Ratamess NA,
Alvar BA, Evetoch TK, Terry J. Housh
TJ, Kibler WB et al. American College of Sports
Medicine position stand. Progression models in resistance training for healthy
adults. Med Sci Sports Exerc. 2012;41(3). doi: 10.1249/MSS.0b013e3181915670
- Centner C, Wiegel P, Gollhofer A, König D.
Effects of blood flow restriction training on muscular strength and hypertrophy
in older individuals: a systematic review and meta-analysis. Sport Med.
2019;49(1). doi: 10.1007/s40279-018-0994-1
- Clarkson MJ, May AK, Warmington
SA. Is there rationale for the cuff pressures prescribed for blood flow
restriction exercise? A systematic review. Vol. 30, Scandinavian Journal of
Medicine and Science in Sports. Blackwell Munksgaard;
2020;1318–36. doi: 10.1111/sms.13676
- Hughes L, Paton B, Rosenblatt B, Gissane
C, Patterson SD. Blood flow restriction training in clinical musculoskeletal
rehabilitation: A systematic review and meta-analysis. B J Sports Med.
2017.doi: 10.1136/bjsports-2016-097071
- Patterson SD, Hughes L, Warmington
S, Burr J, Scott BR, Owens J, et al. Corrigendum: Blood flow restriction
exercise: considerations of methodology, application, and safety. Frontiers in
Physiology. 2019;10:533. doi: 10.3389/fphys.2019.00533.
- Barber-Westin S, Noyes FR. Blood flow–restricted
training for lower extremity muscle weakness due to knee pathology: a
systematic review. Sports
Health. 2019;21.
- Álvarez
CB, Santamaría PIK, Fernández-Matías
R, Pecos-Martín D, Achalandabaso-Ochoa A, Fernández-Carnero S, et al. Comparison of blood flow
restriction training versus non-occlusive training in patients with anterior
cruciate ligament reconstruction or knee osteoarthritis: A systematic review. J
Clin Med. 2020;10(1):68. https://doi: 10.3390/jcm10010068
- Charles D, White R, Reyes C, Palmer D. A systematic
review of the effects of blood flow restriction training on quadriceps muscle
atrophy and circumference post ACL reconstruction. Int J Sports Phys Ther. 2020;15(6):882-891. doi:
10.26603/ijspt20200882
- Murray J, Bennett H, Boyle T, Williams M, Davison K.
Approaches to determining occlusion pressure for blood flow restricted exercise
training: Systematic review. J Sports Sci. 2021;39(6):663-672. doi:
10.1080/02640414.2020.1840734
- Van Cant J, Dawe-Coz A, Aoun E, Esculier JF. Quadriceps
strengthening with blood flow restriction for the rehabilitation of patients
with knee conditions: A systematic review with meta-analysis. J Back Musculoskelet
Rehabil. 2020;33(4):529-44. doi:
10.3233/BMR-191684
- Lorenz DS, Bailey L, Wilk KE, Mangine
RE, Head P, Grindstaff TL, et al. Blood flow
restriction training. J Athl Train.
2021;56(9):937-944. doi: 10.4085/418-20
- Maher CG, Sherrington C, Herbert RD, Moseley AM,
Elkins M. Reliability of the PEDro scale for rating
quality of randomized. 2003;68:713–21.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. doi: 10.1002/9781119536604
