Fisioter Bras. 2023;24(6):998-1008
REVIEW
Effectiveness and safety of the use of room air
compared to 100% oxygen in neonates under cardiac arrest at birth: systematic
review protocol
Efetividade
e segurança do uso do ar ambiente em comparação ao oxigênio a 100% nos neonatos
em parada cardíaca: protócolo de revisão sistemática
Benedita
Monique Almeida Freires1, Elayne Carvalho
Santos1, Ingrid Nazaré Lourinho Alves1, Fernanda
Gabriella de Siqueira Barros Nogueira1, Juliana Anézia Rodrigues do
Amaral1, Ana Carolina Pereira Nunes Pinto1,2,3, Natalia
Camargo Rodrigues Iosimuta1
1Federal
University of Amapá, Macapá,
AP, Brazil
2Iberoamerican
Cochrane Centre - Biomedical Research Institute Sant Pau (IIB Sant Pau),
Barcelona, Spain
3Cochrane
Brazil, Center for Evidence-Based Health Studies and Health Technology
Assessment, São Paulo, SP, Brazil
Received: August 13, 2023; Accepted:
November 24, 2023.
Correspondence: Ana Carolina Pereira Nunes Pinto,
anacarolinapnp@hotmail.com
How to cite
Freires BMA, Santos EC, Alves INL, Nogueira FGSB, Amaral JAR, Pinto ACPN, Iosimuta NCR. Effectiveness and safety of the use of room air compared to 100% oxygen in neonates under cardiac arrest at birth: systematic review protocol. Fisioter Bras. 2023;24(6):998-1008. doi: 10.33233/fb.v24i6.5522
Abstract
Introduction: Cardiac arrest
at birth represents a serious problem worldwide. The commonly recommended
practice for resuscitation of asphyxiated newborns has been the use of 100%
oxygen for assisted ventilation. However, there is increasing evidence that
ambient air is as effective as 100% oxygen and that 100% oxygen can have
adverse effects on respiratory physiology and cerebral circulation. Objective:
To compare the effectiveness and safety of using room air versus 100% oxygen in
the resuscitation of neonates with cardiac arrest at birth. Methods: For
this purpose, we will perform a systematic review of randomized controlled
trials (RCTs). We will register the study protocol in the Prospero Platform. We
will include neonates of any gestational age, diagnosed with cardiac arrest or
neonatal asphyxia. We will perform searches in the following databases: Medical
Literature Analysis and Retrieval System Online (Medline) via Pubmed, Excerpta Medica dataBASE (Embase) via Elsevier, Cochrane Central Register of
Controlled Trials (CENTRAL) via Cochrane Library, Latin American Literature and
the Caribbean in Health Sciences (Lilacs) via the Virtual Health Library
Portal, with no restrictions on language or year of publication. The selection
of studies, data extraction, assessment of bias of included studies and
assessment of certainty of evidence will be performed by two independent
researchers. Expected Results: We expect to clarify the effectiveness
and safety of using ambient air compared to 100% oxygen, to provide useful
information for clinical decision-making, and to support future high-quality
randomized clinical trials on the subject
Keywords: heart arrest; cardiopulmonary
resuscitation; infant, newborn; oxygen.
Resumo
Introdução: A parada cardíaca ao nascimento
representa um problema grave em todo o mundo. A prática comumente recomendada
para reanimação de recém-nascidos asfixiados tem sido o uso de oxigênio a 100%
para ventilação assistida. No entanto, há evidências crescentes de que o ar
ambiente é tão eficaz quanto o oxigênio a 100% e que o oxigênio a 100% pode ter
efeitos adversos na fisiologia respiratória e na circulação cerebral. Objetivo:
Comparar a efetividade e segurança do uso do ar ambiente com o oxigênio a 100%
na ressuscitação de neonatos em parada cardíaca ao nascimento. Métodos:
Realizaremos uma revisão sistemática de ensaios clínicos randomizados (ECR) e
registraremos o protocolo do estudo na Plataforma Prospero. Incluiremos
neonatos a termo, diagnosticados com parada cardíaca ou asfixia neonatal. As
buscas serão realizadas nas bases de dados: Medical Literature
Analysis and Retrieval System Online (Medline) via Pubmed,
Excerpta Médica dataBASE
(Embase) via Elsevier, Cochrane Central Register of Controlled Trials
(Central) via Cochrane Library, Literatura Latino-Americana e do Caribe em
Ciências da Saúde (Lilacs)
via Portal da Biblioteca
Virtual em Saúde, sem restrições de idioma ou ano
de publicação. A seleção dos
estudos, extração de dados, avaliação do
viés dos estudos incluídos e avaliação
da certeza da evidência serão realizados por dois
pesquisadores independentes. Resultados
esperados: Esperamos esclarecer a efetividade e segurança do uso do ar
ambiente em comparação com o oxigênio a 100%, fornecendo informações úteis para
a tomada de decisão clínica e para embasar futuros ensaios clínicos randomizados
de alta qualidade sobre o assunto
Palavras–chave: parada cardíaca; reanimação
cardiopulmonar; recém-nascido; oxigênio.
Introduction
Cardiorespiratory arrest stands as
a significant global clinical concern. Marked by the cessation of blood
circulation due to the deficiency or inefficacy of cardiac mechanical functions
[1], this condition results in an estimated fatality count of one million
individuals, with a commensurate number enduring subsequent complications
including encephalopathy, neurodevelopmental retardation, and epilepsy [2].
In the context of cardiorespiratory
arrest, the absence of oxygenated blood perfusion to vital organ systems
precipitates organ dysfunction culminating in death. Within this paradigm, the
administration of supplemental oxygen amid cardiopulmonary resuscitation
endeavors emerges as a logical and promising intervention. This approach, when
coupled with resuscitative maneuvers, stands among the foremost advocated
therapeutic measures within the Neonatal Intensive Care Unit for addressing
such occurrences [3,4].
Notwithstanding its
indispensability to sustenance of life, the administration of oxygen, if
executed improperly, bears the potential for toxicity and the subsequent onset
of severe repercussions. The extent of this toxicity, contingent upon variables
encompassing the absolute pressure of dispensed oxygen, temporal span of
exposure, and the inherent susceptibility of the individual, constitutes a
primary constraint in its application [5]. Elevation of oxygen levels to toxic
thresholds transpires with the emergence of hyperoxia, denoting the inhalation
of elevated fractions of oxygen across protracted intervals or at heightened
concentrations.
Contemporary evidence underscores
the proposition that elevated oxygen administration during resuscitative
efforts elicits an excessive release of oxygen-derived free radicals within the
post-hypoxic phase. This phenomenon bears the potential to induce deleterious
cellular and organ-level impairments. Such empirical insights have catalyzed a
series of investigative endeavors, marked by experimental designs, aimed at
elucidating the comparative efficacy of ambient air during neonatal
resuscitation. Initial inquiries that juxtapose room air against pure oxygen
for the resuscitation of asphyxiated neonates have yielded indications of
parity in their effectiveness [6]. Nevertheless, amassed data highlight a
discernible discrepancy: the cohort resuscitated with room air exhibited a
notably abbreviated time frame for inaugural crying and a briefer duration of
ventilation in achieving a sustained respiratory pattern, relative to the
counterpart group resuscitated with 100% oxygen [7].
Adverse outcomes observed during
the process of resuscitation find their rationale in prior investigations,
which posit a correlation between hyperoxemia and a
spectrum of deleterious repercussions. These encompass a postponement in the
initiation of autonomous respiratory activity, escalated oxygen utilization,
and perturbations in cerebral blood flow dynamics [8]. Nonetheless, a
comprehensive exploration into the primary complexities of
hyperoxygenation-related complications in neonates experiencing cardiac arrest
remains a sparsely traversed domain within the scientific literature.
In the present context, the
assessment of extant empirical insights concerning the outcomes of neonatal
resuscitation involving 100% oxygen in juxtaposition to ambient air assumes
significant pertinence within the domain of pediatric public health. This
evaluation serves a dual purpose: to furnish guidance for contemporary
practices and to furnish a foundational scaffold for prospective inquiries in
this realm. Accordingly, the principal objective of this study is to compare
the effectiveness and safety of using ambient air with the use of 100% oxygen
in cardiorespiratory resuscitation of neonates in cardiac arrest at birth.
Methods
Study design
This systematic review protocol
will follow the Preferred Reporting Items for Systematic Reviews and
Meta-Analysis (PRISMA) recommendations and will be conducted in accordance with
the methodological recommendations of the Cochrane Handbook.
Type of studies
We will include randomized
controlled clinical trials (RCTs) published in full text or only in abstract.
Participants
We will include studies with neonates
at term (born > 37 weeks of gestational age) diagnosed with asphyxia or
cardiac arrest in the neonatal period will be included.
Intervention
We will include studies that
evaluated the use of room air in neonates with cardiac arrest.
Comparison
We will consider as comparison
groups the use of 100% oxygen in neonates with cardiac arrest at birth.
Primary outcomes
To evaluate the effectiveness of room air versus 100%
oxygen, we will assess the following:
- Mortality: at hospital or up to 5 years of age.
- Hypoxic-ischemic encephalopathy (i.
e. a clinical neonatal syndrome resulting from a severe and prolonged episode
of ischemia or hypoxia that occurs before or during the time of delivery).
To evaluate the safety, we will
analyze the following:
- Serious Adverse Events: defined as any untoward
medical occurrence, that results in mortality, risk of death, situations that
require hospitalization or extension of existing hospitalization, significant
or persistent disability, congenital anomaly and clinically significant event.
Secondary outcomes
To evaluate the effectiveness of
room air versus 100% oxygen, we will assess the following:
- Neonatal asphyxia (apgar
1, 5 and 10 minutes) (i. e. a clinical-neurological
syndrome that develops when there is significant tissue hypoperfusion and
decreased uteroplacental blood flow, or hypoxia, characterized by insufficient
oxygen in the tissues.)
- Time to first spontaneous breath (minutes) (i. e. the time required to reach a breathing pattern
without the intervention of the resuscitation team).
- Time to first cry (minutes)
- Duration of newborn resuscitation (minutes) (e. g.
the time to establish heart rate > 100/min).
- Length of stay in Intensive Care Unit and in
hospital
- Length of stay under invasive mechanical ventilation
- Neuropsychomotor
development: Developmental milestones at 18 to 24 months of age including
walking and talking
To evaluate the safety, we will
analyze the following:
- Non-Serious Adverse Events (e. g. hyperemia, edema,
nasal dryness, injuries related to the oxygen supply interface).
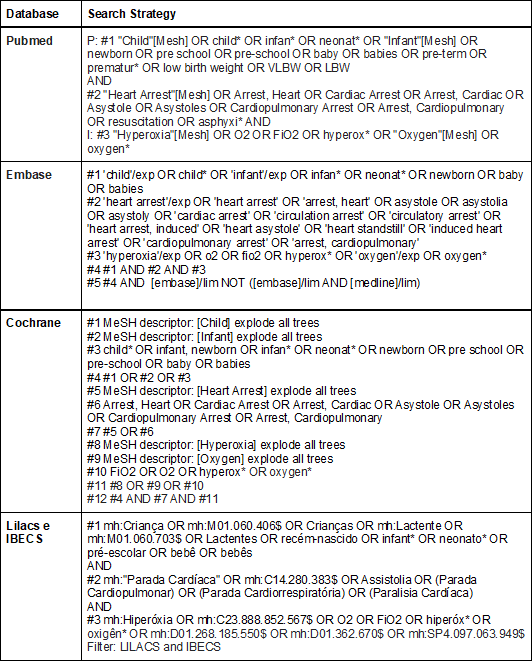
Search and selection of articles
We will carry out sensitive
searches, using pre-specified relevant terms and descriptors, without
limitation of year of publication or language, in the following databases:
- Medical Literature Analysis and Retrieval System
Online (Medline) via Pubmed;
- Excerpta Médica dataBASE (Embase)
via Elsevier;
- Cochrane Central Register of Controlled Trials
(CENTRAL) via the Cochrane Library;
- Latin American and Caribbean Literature in Health
Sciences (Lilacs) and IBECS via the VHL Portal.
Chart 1 - Complete search strategy for
each database
The selection of studies will be
carried out by two completely independent reviewers, based on pre-specified
eligibility criteria. Initially, studies that were indexed in more than one
database (duplicate) will be excluded. After the analysis of potential
duplicates, the evaluation will be carried out based on the titles and abstracts
and, finally, we will read the full texts for further analysis. Disagreements
between authors regarding the inclusion of studies will be discussed by them in
a meeting held after the selection or through analysis by a third reviewer. To
optimize the selection process, we will use the Rayyan application
(https://www.rayyan.ai/). We will present the results related to the study
selection process in a flowchart, as recommended by PRISMA.
Data extraction
We will use a spreadsheet in Microsoft
Excel 365 software to extract the data from the included studies.
Independently, at least two authors (B.M.A.F), (E.C.S) and (I.L.) will extract
the following data: 1) Details regarding the identification (title, authors,
place and date of study); 2) Participants in the experimental and control
groups: number of participants, gestational age, birth weight in grams, number
of cesarean deliveries, general anesthesia, previous pregnancies, labor
induction, aspiration of meconium and number of intubated; 3) Outcomes and
estimates (mean, median, range, standard deviations, 95% confidence intervals,
and number of events).
We will contact the authors of the
studies to clarify any unclear or missing information regarding the evaluated
domain. If the data are insufficient even after contacting the author, the
results of the studies will be summarized only in narrative synthesis.
Risk of bias in each study and assessment of certainty
of evidence
We will perform the assessment of
the risk of bias of the included studies using the tool developed by Cochrane,
called Risk of Bias 2.0 (Risk of bias - ROB 2.0), with the following domains:
bias due to the randomization process, bias due to deviations from intended
interventions, bias due to missing outcome data, bias in measurement of
outcome, bias in selection of reported result, and overall study bias.
We will judge each domain as: low
risk of bias, high risk of bias, or some concern about risk of bias. To
classify the certainty of evidence, we will use the Grading of Recommendations
Assessment, Development and Evaluation System. We will consider factors that
may decrease the certainty in the evidence: (I) the overall risk of bias of the
included studies; (II) the indirectness of the evidence; (III) the
inconsistency of the results; (IV) the precision of the estimates; and (V) the
risk of publication bias.
To summarize judgments about the
certainty of evidence for each main outcome, we will use the GRADE profiler
software, available online (https://gdt.gradepro.org/app/), and will present
the findings in a table with the seven main outcomes, at the longest available
time point:
1. Mortality
2. Hypoxic-ischemic encephalopathy
3. Neonatal asphyxia: apgar
1, 5 and 10 minutes
4. Time of the first spontaneous breath
5. Time to first cry
6. Duration of newborn resuscitation
7. Neuropsychomotor
development
The assessment of the risk of bias
and the certainty of the evidence will be carried out independently by two
evaluators and any disagreements will be resolved through the analysis of a
third examiner.
Statistical
analysis
If at least two studies present sufficient
homogeneity in terms of participants, interventions and evaluated outcomes, we
will pool their results in meta-analyses. When it is possible to carry out
meta-analyses, the data will be grouped using the inverse variance method and
the random effects model in the Review Manager 5.4 software.
When possible, we will synthesize
the continuous variables through the difference in means (post and
pre-intervention) with a 95% confidence interval (CI). In the absence of
results reported as differences in means, or in the absence of good correlation
between individual measurements, the reported data will be used after the
intervention.
We will summarize the dichotomous
variables using relative risk (RR) with a 95% confidence interval (CI). Regarding
dichotomous outcomes, we will consider participants as the unit of analysis,
rather than events (i.e., the number of participants experiencing one or more
adverse events, rather than the number of adverse events per participant). To
assess the variability among studies in each meta-analysis, we will employ the
I2 statistic. In cases of significant heterogeneity (I2 > 50%), we will
explore potential sources of heterogeneity through subgroup or sensitivity
analyses, following the recommendations outlined in the Cochrane Handbook for
Systematic Reviews of Interventions. Additionally, if there is minor clinical
or methodological heterogeneity, we will investigate the sources of
heterogeneity through similar subgroup or sensitivity analyses. Subgroup analysis
will encompass factors such as weight at birth and presence of comorbidities.
We will conduct sensitivity
analyzes, removing studies with a high risk of general bias from the
meta-analyses. If at least 10 studies are included in a meta-analysis, we will
assess the risk of publication bias by analyzing the funnel plot and Egger's
test in the R software (https://www.rproject.org/). When there is a study with
more than two groups, we will only include the relevant arms.
Expected results
Through conducting a thorough
systematic review, our objective is to furnish informative foundations for
clinical practice concerning oxygen administration to full-term neonates
experiencing cardiac arrest. Aligned with the Cochrane methodology, recognized
as the gold standard in the analysis of health intervention studies, this
protocol was rigorously elaborated. We recognize the potential for limitations,
encompassing potential biases in investigations and studies characterized by
restricted sample sizes, which may hinder precise estimations of intervention
outcomes. Nevertheless, this study endeavors to mitigate such constraints by
rigorous methodological, systematic assessment of the certainty of evidence
across diverse outcomes, and comprehensive, judicious literature exploration.
These collective efforts converge to yield a more robust clinical insight, with
a specific aim to elucidate the comparative efficacy and safety of ambient air
versus 100% oxygen in newborns under cardiac arrest at birth. This effort to
clarify things is based on the strongest evidence we have, providing important
insights to support informed clinical decision-making.
Furthermore, the anticipated
outcomes of this protocol possess the potential to expose gaps in existing
knowledge and furnish impetus for future high-quality RCTs within this domain.
Such revelations hold the promise of furnishing indispensable support to
practitioners tasked with the management of neonatal cardiorespiratory arrest,
alongside the endeavor of refining resuscitation guidelines and substantially
enrich and elevate the standards of clinical practice.
Conflitos
de interesse
Não
há.
Fontes
de financiamento
Não
recebido.
Contribuição
dos autores
Concepção
e desenho da pesquisa:
Pinto ACPN, Freires BMA, Santos EC; Coleta de dados: Pinto ACPN, Freires
BMA, Santos EC, Nogueira FGSB, Alves INL; Análise e interpretação dos dados:
Pinto ACPN, Santos ET, Oliveira LL; Análise estatística: Pinto ACPN,
Freires BMA, Santos EC, Nogueira FGSB; Redação do manuscrito: Pinto
ACPN, Freires BMA, Santos EC; Revisão crítica do manuscrito quanto ao
conteúdo intelectual importante: Pinto ACPN, Freires BMA, Santos EC,
Nogueira FGSB, Amaral JAR, Iosimuta NCR.
References
- Matsuno AK. Parada cardíaca em crianças.
Medicina Ribeirao Preto (Online) 2012;45(2):223–33. doi: 10.11606/issn.2176-7262.v45i2p223-233
- Coggins SA, Haggerty M, Heidi MH. Post-cardiac arrest
physiology and management in the neonatal intensive care unit. Ressuscitation. 2021;169:11–19.
doi: 10.1016/j.resuscitation.2021.10.004
- Cruz
VOO, Lanzillotti LS, Zin A,
Entringer AP, Araujo MC,
Silva RCL. Monitorização da oferta do oxigênio suplementar em neonatos:
desafios e potências. Rev Rene. 2019;20. doi: 10.15253/2175-6783.20192041373
- Vento M, Asensi M, Sastre J, García-Sala F, Pallardó
FV, Viña J. Resuscitation with room air instead of
100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001 Apr;107(4):642-7. doi: 10.1542/peds.107.4.642
- Camargo
PAB, Pinheiro AT, Hercos ACR, Ferrari GF.
Oxigenoterapia inalatória em pacientes pediátricos internados em hospital
universitário. Rev Paul Pediatr
2008;26(1):43-7.
- Ramji S, Rasaily R,
Mishra PK, Narang A, Jayam S, Kapoor AN, Kambo I, Mathur A, Saxena BN. Resuscitation of asphyxiated newborns with room air or
100% oxygen at birth: a multicentric clinical trial. Indian Pediatr.
2003 Jun;40(6):510-7.
- Vento M, Asensi M, Sastre J, Lloret A, García-Sala
F, Viña J. Oxidative stress in asphyxiated term
infants resuscitated with 100% oxygen. J
Pediatr. 2003;142(3):240-6. doi:
10.1067/mpd.2003.91
- Rodrigues FPM. Importância dos radicais livres de oxigênio no período neonatal. J Pediatr. 1998;74(2):91-8.
