Fisioter Bras.
2023;24:(4):506-18
REVIEW
Cognitive function, eating behavior and neuroimaging
studies in obese: a systematic review
Função
cognitiva, comportamento alimentar e neuroimagem em obesos: uma revisão
sistemática
Jaqueline
Peixoto Lopes1, Emanoele Anastácia da
Silva Araujo Melo1, Frederico Barreto Kochem2,3,
Ana Carolina Nader Vasconcelos Messias4, Marco Orsini5, Victor
Hugo do Vale Bastos6, Julio Guilherme
Silva1,3, Cristiane Sousa Nascimento Baez
Garcia7, Luciana Moisés Camilo1,7, Mauricio de Sant Anna
Junior1,7
1Instituto de Biofísica Carlos Chagas
Filho, Universidade Federal do Rio de Janeiro (MPT - IBCCF UFRJ), Rio de
Janeiro, RJ, Brazil
2Centro Universitário Anhanguera de
Niterói, Rio de Janeiro, RJ, Brazil
3Universidade Federal do Rio de Janeiro
(UFRJ), Rio de Janeiro, RJ, Brazil
4Hospital Federal dos Servidores do
Estado, Rio de Janeiro (HFSE), RJ, Brazil
5Universidade Iguaçu (UNIG), RJ, Brazil
6Universidade Federal do Delta do
Paranaíba, Parnaíba, PI, Brazil
7Instituto Federal de Educação, Ciência e
Tecnologia do Rio de Janeiro (IFRJ), RJ, Brazil
Correspondence: Mauricio de Sant’Anna Jr, mauricio.junior@ifrj.edu.br
How to cite
Lopes JP, Melo EASA, Kochem
FB, Messias ACNV, Orsini M, Bastos VHV, Silva JG, Garcia
CSNB, Camilo LM, Sant Anna Junior M. Cognitive function, eating behavior and
neuroimaging studies in obese: a systematic review. Fisioter Bras. 2023;24(4):506-18. doi: 10.33233/fb.v24i4.5528
Abstract
Background: Obesity is a
multifactorial disorder influenced by hormonal, dietary, behavioral, emotional,
attentional, and cognitive control factors that interfere with the balance
between energy intake and expenditure. The association of obesity with
cognitive decline, brain functional and structural damage, and early
neurodegenerative processes has been observed. Objective: This
systematic review aimed to identify activated cortical areas in obese
individuals and investigate the role of cognitive impairment in interfering
with eating behavior. The most frequently used neuroimaging and brain mapping
methods to evaluate these processes were also identified. Methods: We
searched for studies published between 2006 and 2021 in the indexed databases
PUBMED, LILACS, and SCIELO. Observational studies that compared obese
individuals (body mass index > 30 kg/m²) and nonobese individuals were
selected. The National Heart, Lung, and Blood Institute (NIH) Quality
Assessment of Observational Cohort and Cross-sectional Studies was used for
methodological quality analysis. Results: The literature search returned
22,484 relevant titles. After applying the eligibility criteria, 154 articles
were selected, and of these, 11 were analyzed in this review. In the analysis,
the groups studied showed differences in reaction time, accuracy, or
inactivated brain areas during tests or stimulation with food images. Conclusion:
Structural changes compatible with impairments in long-term cognitive
performance were identified, as well as structural and functional changes that
may help understanding the compulsive eating behavior present in obese
individuals.
Keywords: obesity; electroencephalogram;
cognition; functional magnetic resonance imaging.
Resumo
Introdução: A obesidade é considerada uma desordem
multifatorial influenciada por fatores hormonais, dietéticos, comportamentais,
emocionais, atencionais e controle cognitivo que interferem no equilíbrio
ingestão e gasto energético. A influência da obesidade no declínio cognitivo e
prejuízos a funções e estruturas cerebrais além de sua associação com processos
neurodegenerativos precoces tem sido observada. Objetivo: Esta revisão
buscou identificar as áreas corticais mais ativadas em indivíduos obesos,
investigar a existência de comprometimento cognitivo e a possível interferência
no comportamento alimentar. Além disso, buscou-se identificar os métodos de
neuroimagem mais utilizados para avaliação desses processos. Methods: Buscou-se estudos publicados 2006 e 2021.
Foram pesquisadas as bases de dados indexadas PUBMED, LILACS e SCIELO. Foram
selecionados estudos observacionais que comparassem indivíduos obesos (IMC >
30 kg/m²) e não obesos. Foi utilizado o Quality
Assessment of Observational
Cohort and Cross-Sectional Studies da National Heart, Lung and Blood Institute
(NIH) para análise de qualidade metodológica. Resultados: Foram
reportados 22.484 títulos. Após a aplicação dos critérios de elegibilidade,
foram selecionados 154 artigos. Desses, onze foram incluídos para análise nesta
revisão. Nesta análise, diferenças foram encontradas quanto ao tempo de reação,
acurácia ou áreas cerebrais inativadas durante os testes cognitivos ou
estímulos com figuras de comida entre os grupos estudados. Conclusão:
Mudanças estruturais compatíveis com prejuízos na performance cognitiva a longo
prazo foram identificadas, assim como alterações estruturais e funcionais que
podem auxiliar o entendimento de comportamento alimentar compulsivo presente em
indivíduos obesos.
Palavras-chave: obesidade; eletroencefalograma;
cognição; ressonância magnética funcional
Introduction
Obesity has become the subject of
numerous studies globally due to its high prevalence in the world population [1],
making this disease an epidemic. Data from the World Health Organization (WHO)
point to obesity as one of the world’s biggest public health problems. In 2016,
1.9 billion adults were overweight and 650 million were obese [2]. According to
the WHO, obesity is defined as the excessive accumulation of body fat and is
measured by the body mass index (BMI). BMI is obtained by dividing an
individual’s mass by the square of height and is expressed in kg/m2
[3]. Using the BMI, obesity can be classified as class I (BMI 30–34.9 kg/m²),
class II (BMI 35–39.9 kg/m²), and class III (BMI ≥ 40 kg/m²) [4]. A
multifactorial disorder, obesity is influenced by hormonal, dietary,
behavioral, emotional, attentional, and cognitive control factors that
interfere with the balance of energy intake and expenditure [5]. It is
associated with several comorbidities, such as type 2 diabetes mellitus,
cardiovascular diseases, metabolic syndrome, and some forms of cancer, and
increases the risk of premature mortality [6,7,8].
In recent years, studies [9,10] on
obesity have sought to elucidate the interactions between homeostatic control
and the neural networks involved in controlling food intake [11], such as
behavior, cognitive factors, social habits, and hedonic appetite control [12,13].
Neuroimaging and brain mapping methods have been widely used to assess the
neurofunctional changes in obese individuals, such as the cortical processes
involved in dietary control and weight gain [1,11,14]. Several authors have
reported limbic and prefrontal neural network dysfunction in obesity,
suggesting that eating behavior can be influenced by the relationship between
reward circuits and cognition [15].
Obesity increases the risk of
cognitive decline, impairment of brain function, and structural damage [16],
independent of its association with early neurodegenerative processes [17].
Neuroimaging has revealed that structural changes related to high BMI are
primarily reductions in gray matter volume in the temporal, frontal, and
occipital lobes [18]. Recent evidence has demonstrated5 greater reductions in
volume in cognitive brain regions, such as the hippocampus, prefrontal cortex,
and anterior cingulate cortex, in obese individuals. Therefore, this systematic
review aimed to evaluate the methodological quality of studies that
investigated the changes in cortical activity and cognitive function in obese
individuals and the possible interference of these changes with eating
behavior.
Methods
To carry out this systematic
review, we followed the guidelines and search principles of the PRISMA
recommendation [19].
Search strategy
The literature search was performed
from May 2019 to October 2022. Studies published between 2006 and 2021 were
searched in the indexed databases PUBMED, LILACS, and SCIELO. The keywords used
in the search process were “obesity” in association with the terms “brain mapping,”
“neuroimaging,” “electroencephalography,” “magnetoencephalography,” “functional
magnetic resonance imaging,” “positron emission tomography,” “single-photon
emission computed tomography,” “pharmacogenetic functional magnetic resonance
imaging fMRI and functional near-infrared spectroscopy,” and “diffusion tensor
imaging”; as well as their association with the term “obese,” in all search
fields (Table I).
Table I - Search strategies for indexed
bases
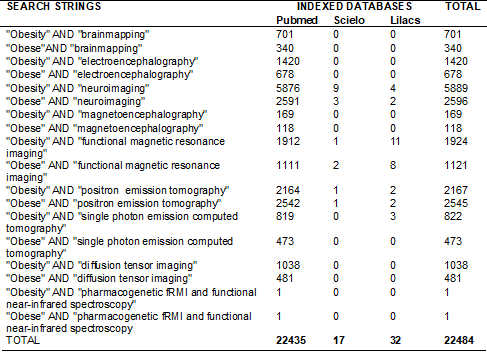
Eligibility criteria
The eligibility criteria used to
include the studies in this review comprised cross-sectional studies in adults
(aged 18 to 60 years), which compared obese (BMI > 30 kg/m²) and nonobese
individuals. Data collected in obese individuals were independently considered
from data in overweight individuals without associated eating disorders.
Studies published in Portuguese, English, Spanish, Italian, and French were
included. Manuscripts covering benign and malignant neoplasms, sleep disorders,
drug intervention, genetic analyzes exclusively, neuropsychiatric disorders,
intervention studies with weight loss, and Prader–Willi syndrome were excluded.
Studies with only the abstracts accessible were excluded.
Selection of studies
The initial search returned a total
of 22,484 published studies. Of these, 639 reports were identified as
potentially relevant based on their abstracts, with 173 duplicate reports
excluded. In the next stage, the 466 abstracts selected were analyzed by
applying the eligibility criteria. Of these, 310 articles did not meet the
inclusion criteria for the following reasons: use of animal models (n = 12),
children and adolescents participants (n = 17), participants with BMI less than
30 kg/m2 (n = 72), elderly participants over 60 years old (n = 6),
obesity associated with other pathologies such as neuropsychiatric illnesses
and binge eating (n = 96), and studies that did not compare groups, evaluated
interventions for weight loss as an outcome, or did not present neuroimaging or
cognitive function assessments (n = 107). Finally, 154 studies with full texts
were analyzed. Of these, 11 studies were selected to assess methodological
quality and included in this review. Two reviewers assessed all internships
independently.
Data extraction and quality assessment
The authors’ names, year of
publication, country, sample size, the cognitive tests used, the neuroimaging
methods, and the associations between cognitive function and behavior were
extracted from the selected studies.
The methodological quality
assessment was performed using the National Heart’s Quality Assessment of
Observational Cohort and Cross-sectional Studies, Lung and Blood Institute
(NIH). This scale was developed by a group of researchers from the National
Heart, Lung, and Blood Institute and the Research Triangle Institute
International to assess the quality of methods, concepts, and other tools [20].
The scale assesses 14 items to evaluate the clarity of objectives, the definition
of the population, selection criteria, clarification of the sample’s
statistical power, use of regression methods, and blinding of the evaluators.
Each item was answered with “YES,” “NO,” or “NO DETERMINED / NOT REPORTED /
DOES NOT APPLY.” Reviewers rated the quality of the studies as “Good,” “Fair,”
or “Poor” [21]. The studies evaluated with “YES” to more than 50% of the items
were classified as “Good.” These studies had the lowest risk of bias and the
results were considered valid. The studies with “YES” between 25% and 50% of
the items evaluated were classified as “Fair.” These studies were susceptible
to some biases that were insufficient to invalidate their results. The “Fair”
quality category is likely to be broad; thus, studies with this rating varied
in strengths and weaknesses. The studies with “YES” below 25% of the items
evaluated were classified as “Poor.” This rating indicated a significant risk
of bias.
The methodological quality of each
study was independently assessed by two evaluators. Disagreements were
subsequently discussed item by item to reach a consensus between the
evaluators.
Results
A total of 22,484 citations were
reported, with 11 studies selected for qualitative analysis after application
of the eligibility criteria. All selected studies were cross-sectional, with a
total population of 508 participants and 198 obese individuals from Germany,
Finland, the United States, Spain, South Africa, and the Netherlands (table II).
Table II - Included articles description
and methodological quality assessment
Of the studies, five showed “Fair”
quality according to the Quality Assessment of Observational Cohort and
Cross-sectional Studies. The main biases identified were sample size,
statistical power, and blinding of the evaluators. Six studies were classified
to have “Good” quality (Table II). Four studies used functional magnetic
resonance imaging (fMRI) as an imaging method [22,23,24,25], three used MRI
associated with diffusion tensor imaging (DTI) [26,27,28], and one used positron
emission tomography (PET) [29]. Two studies used electroencephalography (EEG) [30,31]
and one used magnetoencephalography (MEG) [32]. All selected studies investigated
brain areas activated during a task that assessed cognitive function through
executive function [25,26,27,28,29], reaction time and accuracy [22,24,25,26,27,28,30,31],
and memory [25,28,29,32]. Tuulari et al. [22]
used imagery and cognitive control over food images.
The studies analyzed found
differences in reaction time, accuracy, or inactivated brain areas (frontal
cortex, mainly supplemental and prefrontal motor area, insula, and putamen)
during tests or stimuli with food images between the groups studied. However,
four of the included studies did not find any statistically significant differences
in the cognitive tests [23,28,29,30].
Discussion
This systematic review evaluates
the methodological quality of studies that investigated cognitive function in
obese individuals and its possible interference with eating behavior.
Neuroimaging and brain mapping methods allowed the identification of the
cortical areas that were more activated when obese individuals were subjected
to stimuli of pleasurable foods. These data show the cortical regions involved
in control and eating behavior. After identifying the most activated cortical
areas in the target population, an association between high BMI and reduced
cognitive performance was observed when evaluating executive function, memory,
and attention.
The included studies in this review
assessed cognitive performance using several tests. Although the tests varied
in their specific objectives, these evaluated cognitive functions involved in
decision-making processes, including executive function [23,24,25,27,28,29,30,31],
processing speed [25,26,27,28], memory [25,27,28,29,32], and attention [22,23,24,25,26,30].The
tests showed worsening of cognitive function in obese individuals in seven of
the included studies [22,24,25,26,27,31,32]. Reductions in cognitive performance
observed in obese people are related to changes in brain functions responsible
for the inhibitory control of appetite [32]. Other studies [25] investigated
other changes in neural networks related to obesity using magnetic resonance.
They found an increase in the functional connectivity of external networks
(salience network), mainly in the putamen nucleus, which was related to a lower
speed of mental processing in obese individuals.
Reductions in processing speed may
contribute to overfeeding in obese people through the imbalance between
homeostasis and dietary excesses. Cognition can also be affected by
hypothalamic lesions, as demonstrated in the study by Puiget
et al. [27] Using magnetic resonance, they attributed cognitive changes
and eating disorders to changes in the hypothalamus through their interaction
with the systems that integrate cognition and emotion (hippocampus, amygdala,
and insula). In addition, the hypothalamus is involved in the control of
appetite through the activation of inhibitory and excitatory neurons in the
regulatory systems of food intake [3].
Four studies found no statistical
differences when comparing the cognitive test results between obese and
nonobese participants [23,28,29,30,31]. However, they found changes in dopaminergic
receptors [23,29] and white matter structure [28] using fMRI and cortical
excitation [30] using EEG. In the works by Hendrick et al. [23] and
Volkow et al. [29], obese individuals had lower levels of type 2
dopaminergic receptors (dopamine D2 receptor).
Studies corroborate that obesity is
associated with changes in the function of dopamine and its receptors in the
brain reward circuit. These dysfunctions have been related to changes in
behavior, which can help in understanding the subtypes of obesity [23,24,29,33].
In addition, high BMI values are associated with changes in the metabolism of
the prefrontal cortex and reduced volume of gray matter (Brodmann areas 9, 10,
and 32) [29], as well as changes in white matter [28].
White matter changes involved both
the integrity of the cortical tracts and volume [34]. Obesity is an independent
risk factor for these changes, contributing to cognitive decline. However, our
correspondence with Bolzenius et al. [28]
revealed that in his analysis, a relationship between BMI and impaired cognitive
performance was not observed when controlling age as a confounding factor for
cognitive decline. However, microstructural changes, in addition to
proinflammatory markers and vascular changes, preceded the cognitive changes
(memory and executive function) found in individuals with neurological
disorders, such as dementia and Alzheimer’s disease [26,35]. Impairments in
cognitive performance have also been associated with comorbidities inherent in
obesity, as demonstrated by Bloemendaal et al.
[34]. In this study, obese individuals had alterations in the microstructure of
the white matter that were larger than lean individuals, whereas obese
individuals with type II diabetes had even more significant alterations.
Hume et al. [30] did not
find differences between obese and eutrophic participants in the tests that
measured the reaction time and accuracy of responses in the modified Stroop
task. However, using EEG, they observed greater cortical excitation and
attention during stimulation with food images versus neutral images
(office-related items). Increased reactivity to visual food stimuli can
contribute to hedonic eating and other eating behaviors that lead to weight
gain, such as compulsive eating behaviors [31].
The methods used to analyze
structural changes and cortical excitation in the included studies were fMRI [22,24,25,26,27,28],
PET [29], and EEG [30,31,32]. Magnetic resonance allows the graphical representation
of the activated cortical areas. PET allows the assessment of blood flow and
glucose metabolism, while electroencephalography allows the identification of
the areas with the greatest arousal, attention, and reaction times before
stimuli.
The differences
observed between obese and normal individuals occurred mainly in the frontal
cortex, which governed inhibitory control, emotion, planning, and executive
function, and in the reward circuits [22]. Regions of the frontal cortex,
front-medial, middle-upper and lower gyri, cingulate gyrus, precentral gyrus,
supplementary motor area, thalamus, cerebellum, and occipital cortex were
activated during appetite inhibitory control tasks [22]. These regions
establish neural connections with subcortical regions (amygdala, hypothalamus,
and striatum) and frontocortical regions (motor,
premotor, orbital, and medial prefrontal) that are components of the reward
circuit and are also responsible for appetite control [36].
The subcomponents of the reward
circuit contribute to the processing of external information. In the case of
obese individuals, this circuit exhibited dysfunctions of activity in response
to pleasurable foods, a response similar to substance-dependent individuals [36,37].
The differences found in the neural substrates involved in appetite control and
their diversity of responses, mainly to stimuli of pleasurable foods, can
explain the various manifestations of obesity [24,38].
The diversity in the manifestations
of obesity contributes to its understanding. However, it can be a confounding
factor in the analysis of the results. Therefore, a well-characterized
population was a strong point in our study but is also a limitation as we only
included obese people with a BMI above 30 kg/m2 and excluded
overweight individuals. The included studies showed poor or good quality. This
limited quality of the studies was due to methodological failure in blinding
the assessors, justification of sample size, power description, or effect
estimates. However, our study included only observational studies. This
selection criterion may have restricted our results. Moreover, observational
studies reduce the cause–effect relationship. We recommend further studies with
a well-characterized obese population, distinct from overweight individuals.
Conclusion
The studies included in this review
showed an association between high BMI and reduced cognitive performance,
particularly executive function, and structural and functional changes in the
cerebral cortex involved in reward and inhibitory appetite control. These
changes suggest a relationship between compulsive behavior and impairment of
appetite control in obese people. Both structural and functional changes were
measured using methods analyzing brain signal uptake and brain mapping. The
neuroimaging and mapping methods used were functional magnetic resonance
associated with PET and diffusion tensor imaging, electroencephalography, and
magnetoencephalography, with fMRI being the most frequently used method.
Despite the relevance of the results found, methodological limitations
compromise the quality of the information obtained.
We recommend further studies to
better understand the alterations in the neural circuits in a
well-characterized obese population (BMI above 30 kg/m2). Subgroups in this
population can be evaluated to further correlate BMI with changes in cortical
activity and appetite control behaviors.
Conflict of interests
The authors have no conflicts of
interest to declare.
Funding
Study not funded
Author contributions
L.J. and S.M. conceived of the
review, identified and interpreted relevant studies for inclusion, and wrote the
manuscript. L.J. and K.F. independently assessed the methodological quality of
the studies. All the authors critically revised the manuscript. All the authors
approved of the final manuscript and agreed to be accountable for all aspects
of the work. All persons designated as authors qualify for authorship, and all
those who qualify for authorship are listed.
References
- García-García I, Narberhaus
A, Marqués-Iturria I et al. Neural responses to
visual food cues: insights from functional magnetic resonance imaging. Eur Eat
Disorders Rev. 21:89-98. doi: 10.1002/erv.2216 [Crossref]
- WHO. World Health
Organization (2016). Obesity and overweight 2016[Internet] [citado
2023 ago 25]. Disponível
em: http://www.who.int/mediacentre/factsheets/fs311/en/
- Farr OM, Li CR, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metabolism. 2016;65(5):699-713. doi: 10.1016/j.metabol.2016.02.002 [Crossref]
- Convit A. Obesity is associated with structural and functional brain abnormalities: where do we go from here? Psychosom Med. 2012;74(7):673-4. doi: 10.1097/PSY.0b013e3182662c56 [Crossref]
- Bocarsly ME, Fasolino M, Kane GA et al. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. PNAS. 2015; 112:15731–36. doi: 10.1073/pnas.1511593112 [Crossref]
- KullmannS, Heni M, Veit R et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015;28(6):1044-50. doi: 10.2337/dc14-2319 [Crossref]
- Driscoll I, Beydoun MA, An Y, Davatzikos C, Ferrucci L, Zonderman AB, Resnick SM. Midlife obesity and trajectories of brain volume changes in older adults. Hum Brain Mapp. 2011;33(9):2204-10. doi: 10.1002/hbm.21353 [Crossref]
- Sant Anna Junior M, Carneiro JRI, Carvalhal RF et al. Cardiovascular autonomic dysfunction in patients with morbid obesity. Arq Bras Cardiol. 2015;105(6). doi: 10.5935/abc.20150125 [Crossref]
- Lizarbe B, Campillo B, Guadilla I, López-Larrubia P, Cerdán S. Magnetic resonance assessment of the cerebral alterations associated with obesity development. J Cereb Blood Flow Metab. 2020;40(11):2135–51. doi: 10.1177/0271678X20941263 [Crossref]
- Stopyra MA, Friederich HC, Lavandier N, Mönning E, Bendszus M, Herzog W, Simon JJ. Homeostasis and food craving in obesity: a functional MRI study. Int J Obes. 2021;45:2464-2470. doi: 10.1038/s41366-021-00920-4 [Crossref]
- Cornier MA, McFadden KL, Thomas EA, Bechtell JL, Eichman LS, Bessesen DH, Tregellas JR. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiol Behav. 2013;110-111:122-128. doi: 10.1016/j.physbeh.2013.01.002 [Crossref]
- Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, TregellasJR. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105:1028-34. doi: 10.1016/j.physbeh.2011.11.023 [Crossref]
- Blechert J, Klackl J, Miedl SF, Wilhelm FH. To eat or not to eat: effects of food availability on reward system activity during food picture viewing. Appetite. 2016;99:256-61. doi: 10.1016/j.appet.2016.01.006 [Crossref]
- Kullmann S, Callaghan MF, Heni M et al. Specific white matter tissue microstructure changes associated with obesity. Neuroimage. 2016;125:36-44. doi: 10.1016/j.neuroimage.2015.10.006 [Crossref]
- Alonso-Alonso M. Translating tDCS into the field of obesity: mechanism-driven approaches. Front Hum Neurosci. 2013;7:1-3. doi: 10.3389/fnhum.2013.00512 [Crossref]
- Figley CR, Asem JSA, Levenbaum EL, Courtney SM. Effects of body mass index and body fat percent on default mode, executive control, and salience network structure and function. Front Hum Neurosci. 2016;10:1-23. doi: 10.3389/fnins.2016.00234 [Crossref]
- Ronan l, Bloch AFA, Wagstyl K et al. Obesity associated with increased brain age from midlife. Neurobiol Aging. 2016;47:63-70. doi: 10.1016/j.neurobiolaging.2016.07.010 [Crossref]
- Kulmann S, Heni M, FritscheA, Preissl H. Insulin action in the human brain: evidence from neuroimaging studies. J Neuroendocrinol. 2015;21;419-23. doi: 10.1111/jne.12254 [Crossref]
- Galvão TF, Pansani TSA. Principais itens para relatar revisões sistemáticas e meta-análises: a recomendação PRISMA. Epidemiol Serv Saúde. 2015;24:335-42. doi: 10.5123/S1679-49742015000200017 [Crossref]
- Quality assessment tool for observational cohort and
cross-sectional studies – NHLBI, NIH. Disponível
em: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Maass SWMC, Roorda C, Berendsen AJ, Verhaak PFM, Bock GH. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015;82(1):100-8. doi: 10.1016/j.maturitas.2015.04.010 [Crossref]
- Tuulari JJ, Karlsson HK, Hirvonen J, Salminen P, Nuutila P, Nummenmaa L. Neural circuits for cognitive appetite control in healthy and obese individuals: an fmri study. PLoS One. 2015;10(2):e0116640. doi: 10.1371/journal.pone.0116640 [Crossref]
- Hendrick OM, Luo X, Zhang S, Li CR. Saliency processing and obesity: a preliminary imaging study of the stop signal task. Obesity. 2015;20:1796–802. doi: 10.1038/oby.2011.180 [Crossref]
- Balodis IM, Molina ND, Kober H et al.Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity. 2013;21:367-77. doi: 10.1002/oby.20068 [Crossref]
- García-García I, Jurado MA, Garolera M et al. Alterations of the salience network in obesity: aresting-state fmri study. Hum Brain Mapp. 2012. doi: 10.1002/hbm.22104 [Crossref]
- Fernandez-Real JM, Serino M, Blasco G et al. Gut microbiota interacts with brain microstructure and function. J Clin Endocrinol Metab. 2015; 100: 4505–13. doi: 10.1210/jc.2015-3076 [Crossref]
- Puig J, Blasco G, Daunis-i-Estadella J, et al. Hypothalamic damage is
associated with inflammatory markers and worse cognitive performance in obese
subjects. J Clin Endocrinol Metab. 2015;100:
E276–E281. doi: 10.1210/jc.2014-2682 [Crossref]
- BolzeniusJD,Laidlaw DH, Cabeen RP, et al. Brain structure and cognitive correlates of body mass index in healthy older adults. Behav Brain Res. 2015;278: 342–47. doi: 10.1016/j.bbr.2014.10.010 [Crossref]
- Volkow ND, Wang GJ, Telang F. Inverse association between bmi and prefrontal metabolic activity in healthy adults. Obesity. 2008;17:60–65. doi: 10.1038/oby.2008.469 [Crossref]
- Hume DJ, Howells FM, Rauch HGL, Kroff J, Lambert EV. Electrophysiological indices of visual food cue-reactivity.Differences in obese, overweight and normal weight women.Appetite. 2015; 85: 126–37. doi: 10.1016/j.appet.2014.11.012 [Crossref]
- Nijs IMT, Franken IHA, Muris P. Food-related stroop interference in obese and normal-weight individuals: behavioral and electrophysiological indices. Eat Behav. 2010;11:258–65. doi: 10.1016/j.eatbeh.2010.07.002 [Crossref]
- Stingl KT, Kullmann S, Ketterer C, Heni M, Häring HU, Fritsche A, Preissl H. Neuronal correlates of reduced memory performance in overweight subjects. NeuroImage. 2012;60:362–69. doi: 10.1016/j.neuroimage.2011.12.012 [Crossref]
- Eisenstein SA, Gredysa DM, Antenor-Dorsey JA, et al. Insulin, central dopamine d2 receptors, and monetary reward discounting in obesity. PLoS One. 2015. doi: 10.1371/journal.pone.0133621 [Crossref]
- Bloemendaal L, Ijzerman RG, Kulve JS, Barkhof F, Diamant M, Veltman DJ, Duinkerken E. Alterations in white matter volume and integrity in obesity and type 2 diabetes. Metab Brain Dis. 2016;31:621–29. doi: 10.1007/s11011-016-9792-3 [Crossref]
- Gunstad J, Strain G, Devlin MJ, et al. Improved memory function 12 weeks after bariatric surgery. Surg Obes and Relal Dis. 2011;7:465–72. doi: 10.1016/j.soard.2010.09.015 [Crossref]
- Nummenmaa L, Hirvonen J, Hannukainen JC, Immonen H, Lindroos MM, Salminen P, Nuutila P. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PLoS One. 2012. doi: 10.1371/journal.pone.0031089 [Crossref]
- Ziauddeen H,
Alonso-Alonso M,Hill JO,
Kelley M,Khan NA. Obesity and the neurocognitive
basis of food reward and the control of intake. AdvNutr.
2015;6:474–86. doi: 10.3945/an.115.008268 [Crossref]
- Hendrikse JJ, Cachia RL, Kothe EJ, McPhie S, Skouteris H, Hayden MJ. Attentional biases for food cues in overweight and individuals with obesity: a systematic review of the literature. Obes Rev. 2015;16:424-32. doi: 10.1111/obr.12265 [Crossref]
