Fisioter Bras. 2023;24(6):940-49
RELATO DE CASO
Limb-girdle muscular dystrophy and physical therapy:
update and case report
Distrofia
muscular de cinturas e fisioterapia: atualização e relato de caso
Marco
Orsini1, Marcela de Moares Mesquita1,
Marcos RG de Freitas2, Clara de Araujo
Leite3, Lara Alexandre Brandão Tomassini4, Thiago
de Mello Tavares5, Victor
Hugo Bastos6, Maria Victórya Manzi de Sant Anna7, Mauricio Sant’Anna Junior8
1Universidade Iguaçu, Nova Iguaçu, RJ, Brasil
2Universidade Federal de Rio de Janeiro, RJ, Brasil
3Universidade Estácio de Sá, Rio de Janeiro, RJ, Brasil
4Instituto de Ressonância Magnética Lara Brandão, Rio
de Janeiro, RJ, Brasil
5Universidade do Contestado, Mafra, SC, Brasil
6Universidade federal do Delta de Parnaíba, PI, Brasil
7Universidade Anhanguera, Niterói, RJ, Brasil
8Instituto Federal de Rio de Janeiro, RJ, Brasil
Received 2023 October
21; accepted 2023 December
12
Correspondence: Marco Orsini,
orsinimarco@hotmail.com
How to cite
Orsini M, Mesquita MM, Freitas
MRG2, Leite CA, Tomassini LAB, Tavares TM, Bastos VH, Sant Anna
MVM, Sant’Anna Junior M. Limb-girdle muscular dystrophy and physical
therapy: update and case
report. Fisioter Bras
2023;24(6):940-49. doi:10.33233/fb.v24i6.5568
Abstract
The term limb girdle muscular dystrophy (LGMD) has
been introduced to delineate a distinct form of muscular dystrophy with
predominantly proximal upper and lower extremity weakness. PAS, 71 years old,
male, retired, diabetic and hypertensive. He reports that the clinical picture
started in mid-2010 with complaints related to atrophy of the shoulder girdle
and thigh region. "When looking at myself in the mirror I started to
notice that I was losing muscle in my shoulders". The beginning of the
clinical picture dragged on, still with muscle shape close to normal to perform
daily activities. He points out that the cervical region bothers him a lot,
mainly due to the inclined position in which he is. In this clinical case, we
present neurological and functional evaluations, as well as complementary exams
that help in the detection of LGMD. New studies, including a larger number of
patients with myopathy of the waist and limbs, are needed. We believe that the
best form of rehabilitation for this group of myopathies is not to look for
overuse damage and, undoubtedly, work in search of function, not primarily
strength.
Keywords: myopathy, rehabilitation; limb
girdle muscular dystrophy
Resumo
O termo
distrofia muscular de cinturas (DMC) foi introduzido para delinear uma forma
distinta de distrofia muscular com fraqueza predominantemente proximal nas
extremidades superiores e inferiores. PAS, 71 anos, sexo masculino, aposentado,
diabéticos e hipertenso. Ele relata que o quadro clínico iniciou em meados de
2010 com queixas relacionadas à atrofia da cintura escapular e região da coxa.
“Ao me olhar no espelho comecei a perceber que estava perdendo músculos nos
ombros”. O início do quadro clínico se arrastou, ainda com formato muscular
próximo ao normal para realização das atividades diárias. Ele ressalta que a
região cervical o incomoda bastante, principalmente pela posição inclinada em
que se encontra. Neste caso clínico apresentamos avaliações neurológicas e
funcionais, bem como exames complementares que auxiliam na detecção de DMC.
Novos estudos, abrangendo um número maior de pacientes com miopatia
de cintura e membros, são necessários. Acreditamos que a melhor forma de
reabilitação para esse grupo de miopatias é não
buscar danos por uso excessivo e, sem dúvida, trabalhar em busca de função e
não principalmente de força.
Palavras-chave: miopatia,
reabilitação; distrofia muscular de cinturas.
Introduction
The term limb girdle muscular
dystrophy (LGMD) has been introduced to delineate a distinct form of muscular
dystrophy with predominantly proximal upper and lower extremity weakness.
Families with evidence of both autosomal recessive and autosomal dominant modes
of inheritance have been described. The recognition of other disorders
presenting with weakness in a limb girdle distribution, such as the spinal
muscular atrophies, dystrophinopathies, inflammatory
and metabolic myopathies, casted doubt on the existence of LGMD as a separate
entity [1-2].
We present a case of muscular
dystrophy of the waist and limbs and, based on the impairments and functional
disabilities resulting from muscle weakness, we point out some proposals for
rehabilitation.
Case Report
PAS, 71 years old, male,retired. Diabetic and
Hypertensive. He reports that the clinical picture started in mid-2010 with
complaints related to atrophy of the shoulder girdle and thigh region.
"When looking at myself in the mirror I started to notice that I was
losing muscle in my shoulders". The beginning of the clinical picture
dragged on, still with muscle shape close to normal to perform daily
activities. He points out that the cervical region bothers him a lot, mainly
due to the inclined position in which he is. Neurological examination: On
inspection: muscle atrophy is noted in the shoulder and pelvic girdles. Muscle
Strength: In the main muscle groups evaluated in the upper and lower limbs, the
predilection for weakness occurred in the proximal third of the brachial and
bilateral crural muscles (figure 1). The most
intermediate and distal segments had muscle strength grade 45 (MRC). Normal
Deep Reflexes. Superficial and deep sensitivity: normal. Cranial Nerve Nuclei:
Normal. Physical function: Height 1.74 (m), weight 77.2 kg, body mass index
(BMI) 25.5 kg/m2, muscle mass 55.1 kg, % fat 23.9, FFM/m2
18.2. Despite presenting functional independence when evaluated by the Barthel
index (scoring 95/100), when performing the Short Physical Performance Battery hort Physical Performance Battery (SPBB) it presented three
points (3/12) characterizing a “low capacity”. After evaluating the peripheral
muscle strength by means of digital dynamometry, it was found that the handgrip
strength was preserved, for the dominant limb reaching 104.2% in relation to
the predicted value (figure 2A), and 119.4% of the predicted value in the
non-dominant limb (figure 2B). After the assessment of inspiratory muscle
strength (MIP) was performed using digital manovacuometry,
it was observed that 91.8% were obtained in relation to the predicted values
(figure 2C). There were no changes in static and/or dynamic balance compatible
with the risk of falling, assessed using the Timed Up and Go (TUG), the patient
completed the course in 9 seconds. Electroneuromyography: myopathic pattern
with axial predominance (Figure 1). MRI of the brachial plexus: denervation
with liposubstitution in the proximal brachial and crural third. Muscle biopsy: Immunohistochemical changes
for dystrophy and sarcoglycans, which were positive,
suggesting myopathy of proximal predominance.It
is noteworthy that the patient has lateral neck tilt due to several inadequate
synergies of movement that he performed for years. Initially, a picture of
dystonia was thought, but the electroneuromyography ruled it out (figure 3). As a result of the thoracolumbar anchorage, we noticed
filiform hydromyelia throughout the entire length of
the medulla (figure 4). The diagnosis of recessive bulbospinal amyotrophy was
excluded.
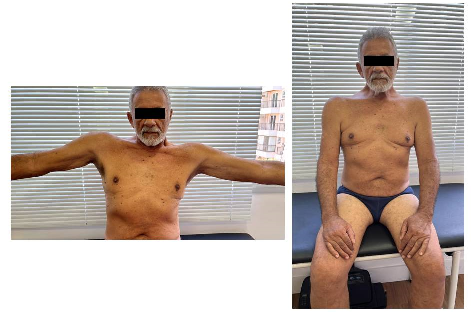
Figure 1 - Note brachial proximal muscle
atrophy and difficulties in raising the arms. Note proximal brachial and crural muscle atrophy

Figure 2 - Handgrip strength (HG) for
the dominant limb (A) non-dominant limb (B), maximal inspiratory pressure – MIP
(C) predicted value in the non-dominant limb (figure 3B). After the assessment
of inspiratory muscle strength (MIP) was predicted vs. obtained
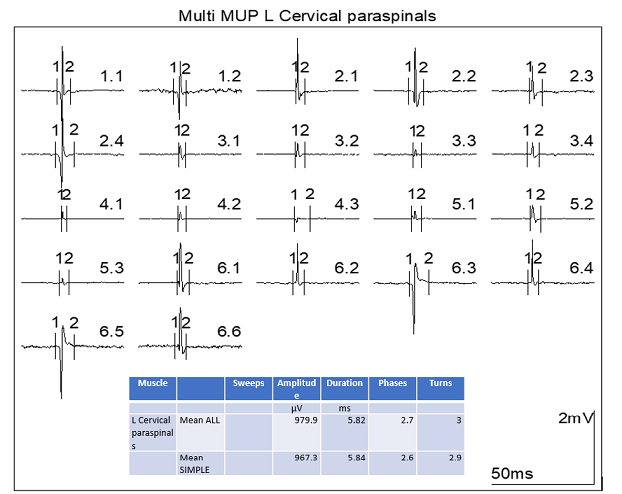
Figure 3 - Quantitative Multi Motor Unit
Potential Analysis of the cervicalparavertebral
musculature showing motor units of reduced duration and amplitude. Indicative
of myopathic disease
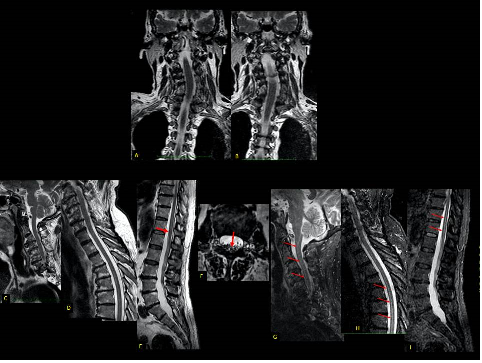
Figure 4 - Neck pain 1 year ago.
Paresthesia in the third and fourth fingers of the right hand. Coronal T2 (A
and B) shows left cervical scoliosis. There are extensive degenerative changes
in the cervical (C), dorsal (D) and lumbosacral (E) spine, with osteophytes in
the vertebrae, degenerative changes in the vertebral plateaus, dehydration and
reduced disc height. Note the stretched and retracted medullary cone posteriorly
inside the dural sac (arrow on E) due to the presence
of a thick terminal filum (arrow on F). There are extensive degenerative
changes in the cervical (C), dorsal (D) and lumbosacral (E) spine, with
osteophytes in the vertebrae, degenerative changes in the vertebral plateaus,
dehydration and reduced disc height: As a consequence of anchoring, we noticed
filiform hydromyelia throughout the entire length of
the medulla (arrows in G-I-sagittal STIR)
Discussion
The spectrum of conditions
encapsulated by this subset ranges from severe and fatal congenital muscular
dystrophies with onset in infancy to mild forms of limb and girdle weakness
with onset in adulthood and minimal respiratory compromise. The list and
classification of muscular dystrophies are undergoing near-constant revision,
based largely on new insights from genetics and molecular medicine [3].These
advances are reflected in the development of new therapeutic approaches, some
of which have already led to clinical trials in the dystrophinopathies
and limb-girdle dystrophies4.We present a case of muscular dystrophy of the
waist and limbs and, based on the physical and neurological examination, we
suggest possibilities for rehabilitation treatment [4].
Cup et al. [5], aimed
through a systematic review to summarize and measure evidence on rehabilitation
for patients with neuromuscular diseases, including myopathies. The search
sources were: Cochrane Central Register of Controlled Trials and Cochrane Database
of Systematic Reviews, Medline, CINAHL, EMBASE (Rehabilitation and Physical
Medicine). Study Selection comprised: Randomized Clinical Trials (RCTs) and
Controlled Clinical Trials (CCTs). The patients in the study had to suffer from
the NMDs listed below: diseases of the motor neuron, motor nerve roots or
peripheral nerves, neuromuscular transmission or muscular diseases
(myopathies). For data extraction, two reviewers decided on the inclusion or
exclusion of articles to evaluating the methodology employed. A level of
evidence was assigned to each subgroup of NMD and each type of intervention.
Initially (data synthesis) 58 studies were included: 12 RCTs, 5 CCTs and 41
other research projects. After the first evaluation pairing, 19 other projects
were excluded. There is level II evidence ("likely to be effective")
for strengthening exercises in combination with aerobic exercises for patients
with muscle disorders. Level III evidence ("indications of efficacy")
was found for aerobic exercise in patients with muscle disorders and for the
combination of muscle strengthening and aerobic exercise in a heterogeneous
group of muscle disorders. Finally, there is level III evidence for breathing
exercises for patients with myasthenia gravis and for patients with myotonic
muscular dystrophy. Adverse effects of exercise therapy were negligible. The
available evidence is limited, but relevant for physicians and professionals
who have little access to therapeutic practices. Multicenter studies, using the
International Classification of Functioning (ICF), will probably improve the
comparability of results5.
Recent studies are in line with
earlier ones further supporting safety and efficacy of exercise in patients
with polymyositis or dermatomyositis. There is an urgent need for larger
randomized controlled trials also including patients with inclusion body
myositis to further increase knowledge of disease mechanisms causing
disability, exercise effects, and what exercise program is most efficient in
patients with different entities of idiopathic inflammatory myopathies [6].
Orsini M et al. [7],
described the case of a patient with mitochondrial myopathy. In addition, they
sought numerous studies involving short-term, moderate-intensity aerobic
training in this myopathy group. The results were satisfactory in the
improvement of respiratory capacity, resistance to fatigue and tolerance in the
execution of basic and instrumental activities of daily living.in patients with
mitochondrial myopathy [7].
Other authors point out that training
programs rehabilitative treatment composed of high-intensity exercises
intensity, although well tolerated by patients with neuromuscular diseases with
weakness (medium to moderate), can cause deleterious effects on the musculature.skeletal striated latature. high resistance exercises do not seem to offer
greater advantages when compared to light to moderate exercises. Exercise-based
training protocols for patients with myopathies of various etiologies should
measure the intensity, duration, frequency and type of activities proposed [8-10].
The basic facilitation procedures
provide tools for the therapist to help the patient gain efficient motor
function and increased motor control. The basic procedures can use to treat
patients with any diagnosis and or condition, although a patient condition may
rule out the use of some of them. Evidence based physiotherapy treatment is
based upon external support of the therapeutic care/intervention combined with
the expertise and experience of the therapist, adapted to the needs and
objectives of the patient. The results presented reinforce that the techniques
of PNF, when employed after a correct kinetic-functional diagnosis, promote
satisfactory results in the management of the muscular weakness and training of
the functional abilities [11-13].
Despite the reports described in
the literature regarding the possible involvement of the respiratory muscles in
patients with limb-girdle muscular dystrophy [14-16], the patient described has
preserved ventilatory musculature. It is recommended that every patient with
muscular dystrophy undergo an adequate respiratory assessment so that any
changes can be identified as soon as possible. The reduction in respiratory
function and respiratory muscle strength and resistance is directly related to
the individual's functional condition.
Another issue that should always be
present in the evaluation of patients with Limb-girdle is the verification of
the presence of sarcopenia [17], a condition that may be superimposed on the
primary clinical condition and lead to functional decline, morbidity and
mortality [18], but the patient presented here does not fulfill sarcopenia
criteria (muscle strength – hand grip, muscle quantity or quality –
bioimpedance, physical performance – TUG) despite having a “low capacity” SPPB.
A fact that deserves to be
highlighted is that although the patient presented a reduction in peripheral
muscle strength assessed by the MRC, this was not demonstrated in the
assessment using the hand grip. For clinical conditions such as the
Limb-girdle, using at least two ways to measure strength can provide important
information regarding the staging of both the clinical and functional condition
[19].
New studies, comprising a larger
number of patients with myopathy of the waist and limbs, are needed. We believe
that the best form of rehabilitation for this group of myopathies is not to
look for overuse damage and, undoubtedly, work in search of function, not
primarily strength.
This work did not receive any
funding and there is no conflict of interest on the part of the authors. All
authors contributed to writing the paper
References
- Taheri F, Taghizadeh E, Pour
MJR, Rostami D, Renani PG, Rastgar-Moghadam
A, Hayat SMG. Limb-girdle muscular dystrophy and therapy: insights into cell
and gene-based approaches. Curr Gene Ther. 2020;19(6):386-94. doi:
10.2174/1566523220666200218113526
- Taheri F, Taghizadeh E, Pour
MJR, Rostami D, Renani PG, Rastgar-Moghadam
A, Hayat SMG. Limb-girdle muscular dystrophy and therapy: insights into cell
and gene-based approaches. Curr Gene Ther. 2020;19(6):386-394. doi:
10.2174/1566523220666200218113526
- Carter JC, Sheehan DW, Prochoroff
A, Birnkrant DJ. Muscular dystrophies. Clin Chest
Med. 2018 Jun;39(2):377-89. doi:
10.1016/j.ccm.2018.01.004
- Kunti C, Ryain A, Dogra P,
Ghosh A, Parab S. Physical therapy-based early
intervention in limb-girdle muscular dystrophy. Indian J Pediatr.
2023 Mar;90(3):308. doi: 10.1007/s12098-022-04444-1
- Cup EH, Pieterse AJ, Ten Broek-Pastoor
JM, Munneke M, van Engelen
BG, Hendricks HT, van der Wilt GJ, Oostendorp RA.
Exercise therapy and other types of physical therapy for patients with
neuromuscular diseases: a systematic review. Arch Phys Med Rehabil.
2007 Nov;88(11):1452-64. doi:
10.1016/j.apmr.2007.07.024
- Alexanderson H. Exercise effects in patients with
adult idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2009 Mar;21(2):158-63. doi:
10.1097/BOR.0b013e328324e700
- Orsini M, Freitas MRG, Mello MP, Reis CH, et
al. Myopathy of atypical form and late-onset (clinical and rehabilitation
aspects): Case report. Rev Neurocienc.
2010;18(2):161-65. doi: 10.34024/rnc.2010.v18.8495
- Ansved T.
Muscle Training in muscular dystrophies. Acta Physiol
Scand 2001;171:359-66. doi: 10.1046/j.1365-201x.2001.00839.x
- Mette C, Orngreen BS, David
B, Olsen MD. Ann Neurol. 2005;57:754-7.
- Orsini M, Freitas MRG, Nascimento OJM.
Precauções na realização de exercícios terapêuticos para pacientes com doenças
neuromusculares. Fisioterapia Ser.
2007;2:272-5.
- Johnson GS, Johnson VS. The application of the
principles and procedures of PNF for the care of lumbar spinal instabilities. J
Manual Manipul Ther. 2002;2:83-105. doi:
10.1179/106698102790819274
- Klein DA, Stone WJ, Phillips W, Gangi
J, Hartman S. PNF training and physical function in assisted living older
adults. J Aging Phys Act. 2002;10:476-88. doi: 10.1123/japa.10.4.476
- Wang RY. The effect of proprioceptive neuromuscular
facilitation in case of patients with hemiplegia of long and short duration.
Phys Ther. 1994;12:25-32. doi: 10.1093/ptj/74.12.1108
- Stübgen JP,
Ras GS, Schultz CM, Crowther G. Lung and respiratory muscle function in limb
girdle muscular dystrophy. Thorax. 1994;49(1):61-5. doi:
10.1136/thx.49.1.61
- Gigliotti F, Pizzi A, Duranti R, Gorini M, Iandelli I, Scano G. Control of
breathing in patients with limb girdle dystrophy: a controlled study. Thorax 1995;50(9):962-8. doi:
10.1136/thx.50.9.962
- Lo Mauro A, Aliverti A.
Physiology of respiratory disturbances in muscular dystrophies. Breathe
(Sheff). 2016;12(4):318-27. doi:
10.1183/20734735.012716
- Sakuma K, A Wataru,
Yamaguchi A. The intriguing regulators of muscle mass in sarcopenia and
muscular dystrophy. Front Aging Neurosci. 2014;29;6:230. doi: 10.3389/fnagi.2014.00230
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T. Sarcopenia: revised European
consensus on definition and diagnosis. Age
Ageing. 2019;48(1):16-31. doi:
10.1093/ageing/afy169
- Diella E, LoMauro A, Delle Fave M, Cima R, Angelo MG. The Performance of Upper Limb (PUL) module in limb-girdle muscular dystrophy. Acta Myol. 2022 Dec 31;41(4):207-11. doi: 10.36185/2532-1900-084
