ARTIGO ORIGINAL
Obtaining and characterization of freeze-dried whole
taro root (Colocasia esculenta),
mucilage and residue as functional food
Obtenção
e caracterização do inhame (Colocasia esculenta) integral, mucilagem e resíduo liofilizados como
alimento funcional
Juliana de Brito Maia
Miamoto, M.Sc.*,
Joelma Pereira, D.Sc.**, Suzan
Kelly Vilela Bertolucci, D.Sc.***
*Nutricionista,
Professora DCA/UFLA, **Professora Titular DCA/UFLA, ***Professora DAG/UFLA. This study is part of
a Master’s thesis with defense on 03/18/2008
Recebido 26 de
janeiro de 2017; aceito 15 de dezembro de 2017
Endereço
para correspondência:
Juliana de Brito Maia Miamoto, DCA/UFLA, Campus
Universitário UFLA, Cx postal 3037 37200-000 Lavras
MG, E-mail: julianamiamoto@uol.com.br; Joelma Pereira: joper@dca.ufla.br; Suzan Kelly Vilela Bertolucci: suzan@dag.ufla.br
Abstract
Taro root (Colocasia esculenta L.),
a starch based plant widely grown for direct consumption, has been produced for
more than 2000 years in regions with a tropical climate. In Brazil, it is a
crop grown by small producers, using it for direct consumption. Some industries
use the tubers for preparation of baby food; however, there is no industrial
processing of taro root. It contains high caloric and protein value and has
elements such as phosphorus and potassium and B-complex vitamins; moreover, in
popular medicine, it has recognized medicinal properties of detoxification,
purification, anti-beriberi properties, etc. The purpose of this study was to
determine, characterize and obtain whole taro root flour and its subproducts (mucilage and residue from mucilage extraction)
by means of physical-chemical analyses, with a view toward discovering its
potential qualities as a functional food. The freeze-dried flours of taro root
had considerable proximate composition with lipid values below wheat flour.
Starch appeared in the three types of taro root flour at significant levels.
The nutritional fiber content was on average 50% greater than the daily
requirements of an individual. The minerals Zn, Fe and Mn
appeared at levels able to meet significant percentages of the daily needs of
children, as well as the vitamin C and β-carotene content. Phytochemical
compounds, saponins, anthocyanins
and polyphenols were present in all the flours. As a result, we concluded that
these taro root flours constitute a viable alternative to production of bakery
products based on their starch content. Whole taro root flours and their subproducts may be considered as having potential for
functional foods due to their vitamin, mineral, protein and phytochemical
content, in addition to their expressive quantity of fiber.
Key-words: chemical composition
of foods, taro root flour, nutritional fiber, vitamin C, β-carotene,
phytochemicals.
Resumo
A raiz de inhame (Colocasia esculenta L.),
planta à base de amido amplamente cultivada para consumo direto, é produzida há
mais de 2000 anos em regiões com clima tropical. No Brasil, é uma cultura
cultivada por pequenos produtores, usando para consumo direto. Algumas
indústrias usam os tubérculos para preparar alimentos para bebês; no entanto,
não há processamento industrial de raiz de inhame. Contém alto valor calórico e
proteico e possui elementos como fósforo e potássio e vitaminas do complexo B;
Além disso, na medicina popular, tem reconhecido propriedades medicinais de
desintoxicação, purificação, propriedades anti-beribéri,
etc. O objetivo deste estudo foi determinar,
caracterizar e obter farinha integral de taro e seus subprodutos (mucilagem e
resíduos da extração de mucilagem). por meio de
análises físico-químicas, com objetivo de descobrir suas qualidades potenciais
como alimento funcional. As farinhas liofilizadas da raiz de inhame tinham uma
composição com
valores lipídicos abaixo da farinha de trigo. O amido apareceu nos três tipos
de farinha de raiz de inhame em níveis significativos. O conteúdo de fibra
nutricional que eles oferecem foi em média 50% maior do que as necessidades
diárias de um indivíduo. Os minerais Zn, Fe e Mn apareceram em níveis capazes
de atender percentuais significativos das necessidades diárias das crianças,
bem como o conteúdo de vitamina C e β-caroteno. Compostos fitoquímicos, saponinas, antocianinas e polifenóis
estavam presentes em todas as farinhas. Como resultado, pode-se concluir que
estas farinhas de raiz de inhame constituem uma alternativa viável à produção
de produtos de panificação com base em seu teor de amido. Farinhas
de raiz de inhame inteiras e seus subprodutos podem ser considerados
como tendo potencial para alimentos funcionais devido ao seu conteúdo
vitamínico, mineral, proteico e fitoquímico, além de
sua expressiva quantidade de fibra.
Palavras-chave: composição química
dos alimentos, farinha de raiz de inhame, fibra nutricional, vitamina C, β-caroteno,
fitoquímicos.
Introduction
Taro root (Colocasia esculenta L.),
belonging to the Araceae family is native to tropical
regions of both hemispheres [1]. The group of raw materials consisting of
roots, tubers and related products is second in volume of production and in
nutritional sustenance in the world, after only cereal crops. Taro root is a
starch-based plant widely grown for direct consumption has been produced for
more than 2000 years in regions with a tropical and subtropical climate. The
mean nutritional chemical composition of taro root is in some cases greater
than that of other tubers, consisting of 72 g 100g-1 of moisture, 23
g 100g-1 of carbohydrates, 1.7 g 100g-1 of protein, 0.2 g
100g-1 of lipids, 35 mg 100 g-1 of calcium, 65 mg 100 g-1
of phosphorus, 1.2 mg 100 g-1 of iron and 4.0g 100-1 of
dietary fiber [2]. In addition, taro root contains on average 30 mg 100g-1
of vitamin A, 0.05 to 0.04 mg 100g-1 of vitamin B1, 0.02 to 0.03 mg
100g-1 of vitamin B2 and 12 to 35 mg 100g-1 of vitamin C.
It is considered to be rich in vitamins A, B1, B2, B5, and C, in minerals such
as chlorine, silicon, phosphorus, aluminum, iron, manganese, potassium and sodium
[3,4]. Starch content is the main quality factor of
taro root, which may vary according to fertilization [5], soil conditions,
climate, stage of maturity at the time of harvest and
plant growing conditions in general.
In medicinal terms, taro root is considered to be a powerful blood
purifier and, according to the National Family Expense Study (Estudo Nacional da Despesa Familiar) undertaken by the IBGE, it is also
recommended for prevention of malaria, dengue fever and yellow fever. It also
strengthens the immune system and increases fertility in women due to the
presence of a constituent which is quite similar to the female hormone [6]. All
parts of the plant may be consumed, the tuber, the leaves and the stems. It is
commonly found in supermarkets and may be consumed cooked, as an alternative to
potatoes, or in the form of purees and creamy soups. After peeling it is white
and has a very firm consistency but after cooking it becomes soft and has a
light bluish hue [7] due to its anthocyanin content.
The development of food products based on traditionally grown tropical
products with cultural appeal, like taro root, has attracted the interest of
rural and industrial producers because it would make an increase in the entire
productive chain possible [8].
Taro root is planted throughout the world and, according to estimates
from the FAO [9](2001), this year 1,464 thousand ha of
taro root were planted, which produced 8,868 thousand tons. In this scenario,
Africa holds sway, being responsible for more than 75% of taro root produced.
Three countries of that continent alone – Ghana, Ivory Coast and Nigeria – are
responsible for 67% of taro root [9].
On the consumption side, rich countries of the so-called First World
stand out, among them Japan and the United States, which concentrate around 80%
of imports. The mean imported volume in the period from 1995 to 2000 stood at
194 thousand tons, with financial operations in the order of US$ 165.6 million
in transactions involving 60 countries [9].
Brazil´s involvement in this international market is very limited (it is
not represented in world production), not taking
advantage of its immense edaphoclimatic capacity for
exploitation of these crops and the innumerable business possibilities that
would come from structured chains of production. Although there are records of
growing of Colocasia
in Brazil from the beginning of colonization, expressive development of this
agribusiness is not observed in national territory.
Domestic production of taro root occurs in south central Brazil,
especially in the state of Rio de Janeiro but the exported volume of this
product, in 2001, reached little more than four thousand tons, in other words,
less than 2% of domestic production [9,10].
According to Lima [11], yams (taro) have various potential uses in
addition to their use “in natura” and as a substrate
for the pharmaceutical industry. Through the lack of greater knowledge in
respect to tubers, especially taro, under all aspects, their industrial use,
beyond food purposes, is very restricted. In Brazil, tubers do not have other
channels of application beyond domestic consumption and animal feed. It is
estimated that less than 5% of Brazilian production is utilized for purposes
other than direct human consumption and feed production [12].
According to Lima [11], bakery, confectioner’s shop and pastry shop
products, ice cream and frozen products, as well as frozen, breaded, ready to
use products, may have improved characteristics through the use of taro root
flours, which serve as functional ingredients since they contain protein, gum
or mucilage.
In the literature, it is possible to find data on preparation of flours
from taro root, which taro root has in abundance, mainly in Africa and the
Caribbean, where there is a market for this product for preparation of yam
(taro) puree, a very popular food [13].
Taro root flour may be added to wheat flour for making bread, or it may
be used in various dishes, sweet or salty. That is because the consumption of
bakery products constitutes an alternative source of vitamins, minerals and
proteins [14].
Lima [11] describes that in addition to the various industrial
possibilities for bread production based on the use of mixed flours, taro root
mucilage has emulsifying power that lends a great deal of softness to bread,
increasing its shelf life.
According to Fonseca [15], the use of freeze-dried taro root in natura and the mucilage of taro root in natura as an additive in manufacturing
bread loaves is viable.
The purpose of this study was to obtain and characterize freeze-dried
whole taro root flour and its subproducts, mucilage
and residue from extraction of taro root mucilage, by means of
physical-chemical analyses, aiming to discover its potential as a functional
food.
Material and methods
The experiment was conducted for the preparation and production of whole
taro root flours and its subproducts, mucilage and
residue from extraction of taro root mucilage, here denominated II, MII, RII –
whole taro root, mucilage of whole taro root and residue of whole taro root,
respectively.
The taro root was obtained from a local fruit and vegetable market and,
so as to obtain the study samples, the tubers obtained were separated, selected
and sanitized in sodium hypochlorite (200 mg L-1) according to the
organogram of project execution (Figure 1). After that, the following flours
were obtained – whole taro root – FTI, taro root mucilage - FMT and residue
from extraction of taro root mucilage - FRT, all of them
lyophilized/freeze-dried.
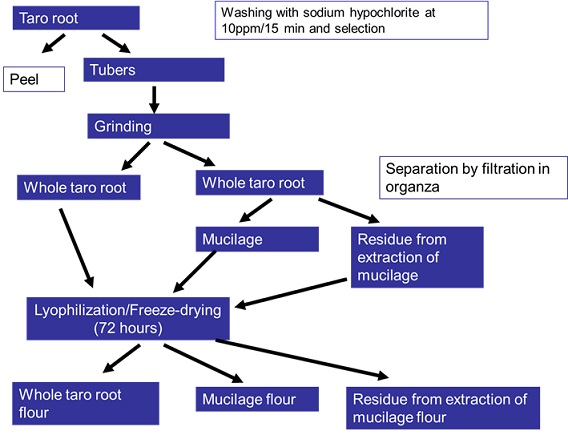
Figure 1 - Organogram of project execution for
obtaining freeze-dried flours of whole taro root, of taro root mucilage and of
residue from extraction of taro root mucilage.
Taro root and its derivative products are rich in water content and, so
as to preserve nutritional properties, the choice was made for freeze-drying to
obtain the flours. Freeze-drying occurred in an Edwards apparatus, model L4KR,
Series 163, for 72 hours or until constant weight, with the material frozen in
a freezer (- 18º C ± x ºC) for 24 hours in Petri dishes covered with plastic
film. Before the mucilage proceeded to the lyophilizer,
the layer of plastic film covering the Petri dish was perforated with around 70
holes by a 0.2mm diameter needle.
Analyses performed on the freeze-dried flours were: moisture (laboratory
oven at 105ºC), ether extract by Soxhlet extraction,
ash, crude protein by the micro-Kjeldahl method
determined according to the methodology proposed by the AOAC [16], crude fiber
through the gravimetric method by Van de Kamer &
Van Ginkel [17], dietary fiber by gelatinization in termamyl according to the AOAC [18]. Fractional glucose was
determined by difference [18], the caloric value by Atwater conversion,
according to the methodology of Osborne & Voogt
[19], pH in digital pH meter and titratable acidity
(AT) according to Cecchi [20], total reducing and
non-reducing sugars carried out by Somogy adapted by
Nelson [21], vitamin C by the colorimetric method [22], the minerals Ca, Mg, Mn, Cu, Zn, Fe, P, S, and
K by Malavolta et
al. [23], the β-carotene where the extract was read at absorbance in
spectrophotometer in accordance with Nagata & Yamashita [24], saponin by standard curve in different concentrations of digitonin (Baccou, Lambert e Sauvaire [25], anthocyanin by Lees & Francis [26],
polyphenols by colorimetry using standard curve by
tannic acid and Folin-Dennis reagent (reagente o Folin-Dennis) [27] and
starch by the standards of the Instituto Adolfo Lutz
[28]. Descriptive statistics was performed in five replicates, obtaining the
mean and standard deviation by means of the software SISVAR 4.03 [29].
Results and discussion
Yield of freeze-dried taro root flours
The taro root tubers in natura yielded 79.30%
pulp and 20.69% peel. The yield of the taro root pulp consisted of 11.62% of
dry matter (DM) and 88.38% moisture. The yield in mucilage and residue was
53.68% of whole mucilage (19.69% DM and 80.31% moisture) and for residue, a
yield of 46.32% (24.20% DM and 76.00% moisture) was found.
Characterization of freeze-dried taro root flours
The mean proximate composition and the caloric value of the freeze-dried
taro root flours are shown in Table I.
Table I - Mean proximate composition plus standard
deviation (moisture, ether extract (E.E.), crude protein, ash, fractional
glucose) and the caloric value of the flours of whole taro root (FTI), mucilage
(FMT) and residue (FRT).
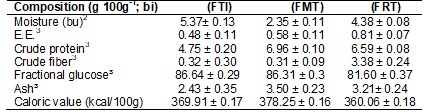
¹Mean
of 5 observations; ²bu = wet basis; ³bi = whole basis
The taro root mucilage flour had the lowest moisture content, followed
by flour from the residue from extraction of taro root mucilage and finally
whole taro root flour. But the moisture content of all the three flours are
within the standards established by Anvisa [30],
which determines that the moisture content of flour should be at most 14%.
Fonseca [15], working with freeze-dried taro root mucilage found 4.36g 100g-1
of moisture (bu), whereas Leonel
et al. [8] in their study with whole
taro root flour dried in a laboratory oven found moisture of 6.2g 100g-1,
both with values greater than those found in this study. Even with the product
undergoing freeze-drying, a percentage of moisture in both flour fractions
(whole taro root, mucilage and residue) was still observed and the same
occurred in the study of Fonseca [15], who also
freeze-dried the mucilage and still found moisture. This occurrence may be
explained because data that determine the time of freeze-drying were not found
in the literature; thus, the principle of the time necessary for obtaining
constant weight was followed.
In regard to the mean content of ether extract, low values were found in
the flours studied. As ether extract is not soluble in water, when separation
of the residue and mucilage was made, it remained concentrated in the residue.
In all the three flours (FTI, FMT and FRT), the lipid content remained below
that found in the wheat flour analyzed by Couto [31].
The data found in the taro root flours were similar to those found by Leonel et al. [8]
in their study of yam flour dried in a laboratory oven, and that of Fonseca
[15] who worked with mucilage flour. Heredia Zárate,
Vieira e Minuzzi [32], for their part, working with
five yam clones and use in home bread baking found 0.71g.100g-1 of
ether extract in yam flour and 1.0g.100g-1 in wheat flour.
The crude protein values found in taro root flours and subproducts (mucilage and residue) were similar to what
Fonseca [15] found in freeze-dried taro root mucilage. Heredia Zárate, Vieira e Minuzzi [32],
for their part, in laboratory oven dried taro root flour found protein content
of 9.04 g 100g-1 in dry basis. Leonel et al. [8] found 5.81 g 100g-1
of crude protein in yam flour in dry basis. Legislation determines that the
minimum protein content in wheat flour must be 11g 100g-1 (bs) [33].
Comparing taro root residue flour (FRT), the crude fiber content was greater
than that found by El-Dash et al.
[34] in whole corn meal (1.2 g 100g-1) and in whole soy flour (3.3 g
100g-1). But what is most interesting in nutritional terms is the
dietary fiber content, which will be discussed further on.
The glucose values found in the present study are greater than those
found by Couto [31](2007) in
wheat flour (85.29 g 100g-1), when compared to freeze-dried whole
taro root flour and mucilage; however, the glucose content in the taro root
residue flour was below that of wheat flour.
According to Brillouet et al. [35] and Ketiku & Oyenuga [36], the stage of maturity is one of the factors
that decisively affect the characteristics of horticulture products. Therefore,
the proximate composition varies in accordance with the physiological stage of
the tubers.
One important quality attribute of food products is determined by the
durability, color, odor, and flavor and they may or should be related to the
acid and organic acid content present in the food. In Table II are shown the
values obtained for pH, titrable acidity (AT) (mEq NaOH 100g-1),
starch and sugars of the freeze-dried flours of whole taro root (FTI), taro
root mucilage (FMT) and residue from extraction of taro root mucilage (FRT).
Table II - Mean values1 of pH, titrable
acidity (meq NaOH 100g-1),
starch and sugars of the freeze-dried flours of whole taro root (FTI), mucilage
(FMT) and residue (FRT) in wet basis.
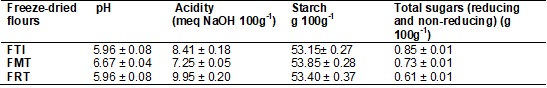
¹Mean of 5 observations.
The greatest value of pH found was in the freeze-dried flour of taro
root mucilage when compared to the others (whole taro root and taro root
residue), but it was in the mucilage flour that the lowest values of titrable acidity were found and in the taro root residue
flour, the highest value. But all three types of freeze-dried taro root flour
are not in accordance with legislation [37] which determines that the flours of
tubers may contain at most 2.0 mL of NaOH/100g (AT).
The data of this study are greater than those found in the literature for wheat
flour, which is the fundamental base for production of bakery products. The
starch content in the flours of taro root and its subproducts,
mucilage and residue from extraction of mucilage, were very similar; however,
it was the flour of the residue from extraction of mucilage that showed the
lowest content of total sugars.
In regard to the total sugar content (reducing and non-reducing),
greater concentration appeared in whole taro root flour; these data are less
than those found by Leonel e Cereda
[38] who found sugars of around 1.19% in wet basis in dehydrated tubers.
Other constituent components of plants are total sugars, starch,
vitamins, and minerals; they mainly make up the nutritional and technological
value of the plants. In Table III are shown the vitamin and mineral values of
the freeze-dried flours of whole taro root (FTI), mucilage (FMT) and residue (FRT).
The mineral and vitamin contents are considered an important quality
attribute of the foods because the food that contains considerable values of
vitamins and minerals is considered to be a “good” food. Mixed flours are
currently being widely studied for the purpose of verifying the aggregation of
nutritional value, especially to bakery products, which have higher per capita consumption in Brazil.
Table III - Mean values1 of macro and
micronutrients (mg kg-1) in whole basis and of vitamins (Vit. C and β-carotene) (mg 100g-1)
in whole basis.
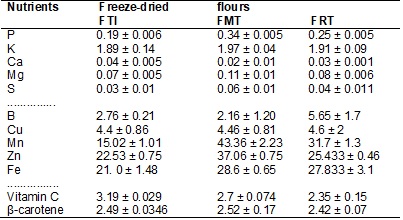
¹Mean of 5 observations.
The mean contents of micronutrients were significant since Brazilian
legislation recognizes and determines the addition of micronutrients, like Fe,
to wheat and corn flours [39] with a view toward decreasing the incidence of anemias in children and pregnant women; thus, bakery
products with the addition of flours that have reasonable contents of this
mineral become significant. The Fe content found in the flours of whole taro
root, mucilage, and residue from extraction of taro root mucilage suggests
fulfilling on average 42.97%, 21.49% and 8.56% of the daily allowances of the
children, adolescent and adult classes according to the RDA [40] respectively.
However, it was mucilage flour and flour of residue from extraction of mucilage
that offered the greatest percentage of iron, capable of offering the
pre-school child (3 to 6 years old) 47.6% of the daily allowance of iron [40].
Zn and Mn appeared with considerable contents
in freeze-dried flours of whole taro root, mucilage and residue from extraction
of taro root mucilage showing that they are capable of meeting on average
94.33%, 35.38% and 25.72% of the daily allowances of Zn for children,
adolescents and adults respectively, but it was flour of taro root mucilage and
flour of residue from extraction of taro root which presented the greatest
percentages of Zn when compared to the daily allowance of this mineral [40 for
children; that is, 100g of use of these flours suggests meeting 123.33%
(mucilage flour) and 84.66% (flour of residue from extraction of taro root
mucilage) of the needs of a child, because the recommended daily allowance
according to the DRIs [41] is 3-5 mg day-1 for children, 8-11 mg dia-1
for adolescents and 11-13 mg dia-1 for adults. The mean content of Mn found in the flours of whole taro root, mucilage and
residue from extraction of taro root mucilage, suggest offers of 3.74%, 1.24%
and 0.93% of the daily needs of children, adolescents and adults respectively
according to the DRI´s [41]. It was mucilage flour and residue from mucilage
extraction flour that suggested offer of the greatest percentages of 5.41% and
3.96% Mn respectively when compared to the needs of a
child [41].
Vitamin C and β-carotene, for their part, appeared in greater
quantities; vitamin C in the whole taro root flour and beta-carotene in
mucilage flour. These vitamins are considered antioxidants and for that reason,
all the flours may be considered as sources because in accordance with the
daily recommendation of the DRIs [42] for vitamin C, the taro root flours and
their subproducts, mucilage and residue, showed an
offer of this vitamin capable of meeting 21.3%, 18% and 15.66% in the flours
respectively when compared to the need of this vitamin for children, which is
15-25 mg day-1; the recommendation of β -carotene is 3.6 to
4.89 mg day-1 for children from 3 to 6 years old [42], and in regard
to β -carotene content, all the taro root flours suggest meeting more than
60% of the daily recommendations for pre-school children.
Dietary fiber is now one of the nutritional components most analyzed and
studied by the scientific community because its activity in the human body is
already well known and approved. The dietary fiber content of the freeze-dried
flours of whole taro root (FTI), mucilage (FMT) and residue (FRT) are shown in
Table IV.
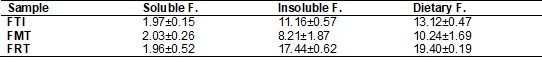
Table IV - Mean contents1of soluble, insoluble and
dietary fiber of taro root flours (FTI, FMT, FRT) g 100 g-1.
¹Mean
of 3 observations
The recommendation of dietary fiber is from 20 to 30g day-1,
with 25% of soluble fiber, which represents 6g day-1 [43]. The mean
contents of freeze-dried flours of whole taro root, mucilage and residue
presented considerable values of dietary fiber in whole materials, with residue
flour presenting the greatest quantities; however, it was in mucilage flour
that soluble fiber showed the greatest concentration, precisely through being
soluble. The freeze-dried flours of whole taro root, mucilage and residue from
extraction of taro root appear to meet 65.6%, 51.2% and 97.0% respectively of
the daily dietary fiber allowance (g 100g-1) of an adult. Heredia Zárate, Vieira e Minuzzi [32]
working with whole yam flour found fiber content of 22.0 g 100g-1 of
dry matter, data similar to those found in the present study. The authors
furthermore commented that through these results, it may be supposed that taro
root is a good dietary alternative for vegetables. Various nutrients are
considered as functional and the mean contents of anthocyanins,
saponins and polyphenols (tannins) analyzed in the
freeze-dried flours of whole taro root, mucilage and residue are shown in Table
V.
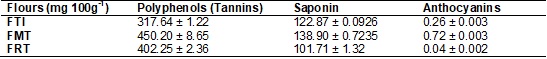
Table V - Mean values1 of polyphenols
(tannins), saponins and anthocyanins
in the freeze-dried flours of whole taro root (FTI), mucilage (FMT) and residue
(FRT).
¹Mean
of 3 observations
According to Schneider [44], Ruiz [45] and Staszewski
& Haenszel [46], taro root contains saponin steroids, principally of the diosgenin
group (dioscin, dioscorin
and others), and Olayemi e Ajaiyeoba
[47] studying Dioscorea extract in mice, having
confirmed the presence of saponins, described in his
biological essay that actually this species of tuber has saponin
contents, confirming anti-inflammatory action. The freeze-dried flours of taro
root have saponin contents, with the greatest saponin content appearing in the mucilage flour; however,
it is good to observe that all three flours showed the presence of saponins.
The polyphenol content (by tannic acid) in the freeze-dried flours of
taro root appeared in greatest quantity in the mucilage flour. The beneficial
effects on health attributed to the polyphenols seem to result from its
antioxidant and free radical scavenging properties, according to Katalinic et al.
[48]. Melo et
al. [49], in their study, consider for verification of content of
polyphenols, carotenoids and flavonoids in normally consumed fruits and
vegetables, that a content > 200 mg 100g-1 of sample is
considered high content, medium content is 100-200 mg 100g and low content is
less than 100 mg 100g-1. Thus, freeze-dried flours of taro root may
be considered as having high polyphenol content.
Anthocyanins are present in taro root tubers [50]. The flours have low
concentrations of anthocyanin content, when compared to the concentrations
obtained from red wines, which range from 30 to 750 mg per 100 g of ripe fruit,
which is considered to be an excellent source of this phytochemical [51]. There
are approximately 400 different anthocyanins, and,
according to Malacrida [52] malvidin-3,5-diglucoside is the type present in grapes, wine, beans
and taro root. But it was freeze-dried flour of taro root mucilage that showed
the greatest contents of this phytochemical.
In spite of not finding the minimum and maximum content of phytochemical
compounds like anthocyanins, saponins
and polyphenols (tannins) in the literature, it could be seen that in all three
types of flour, these compounds appeared in significant quantities. But it was
the taro root mucilage flour that showed the greatest contents.
Conclusion
The flours of whole taro root and its subproducts,
mucilage and residue, showed high percentage content of minerals and vitamins,
suggesting that some, as in the case of Fe, Zn, vitamin C and β-carotene,
were able to offer near or over 25% of the recommended daily allowances, with
mucilage flour in most cases being the greatest holder of these nutrients.
The starch contents were significant in both the
taro root flours and their subproducts, mucilage and
residue, as well as the dietary fiber content; these flours can thus be called
sources of fiber.
Taro root mucilage flour showed the greatest contents of the
phytochemicals analyzed.
It may also be concluded that whole taro root flour and its subproducts can be considered as having potential for
functional food since they have vitamin, mineral, protein and phytochemical
content, as well as an expressive quantity of fiber.
References
- Seagri. Cultura do Cará.
2001. [citado
2007 apr]. Available at:
http://www.seagri.ba.gov.br/cara.
- Woolfe JA. Sweet potato: an untapped
food resource. Cambridge: Cambridge University Press; 1992.
- Araújo FC. de.
Aspectos
sobre o cultivo do inhame-da-costa. Recife: Emater/PE;
1982. 33p. (Boletim Técnico, 29).
- Tecnologias em
agroindústrias de tuberosas tropicais In: Worshop
sobre tecnologias em agroindústrias de tuberosas tropicais. Botucatu/SP: Cerat/Unesp;2004. 181p.
- Oliveira ACB, Seiyama MAM, Sdiyama T et al. Variabilidade genética em batata doce com base em
marcadores isoenzimaticos. Horticulura
Brasileira 2002;20(4):576-82.
- Weil R. As ervas curam. 2 ed. Ground; 1994.
- Herbário. Inhame e
suas características. [cited feb 2007]. Available at:
http://www.herbario.com.br/inhame.
- Leonel M, Mischan MM, Pinho SZ, Iatauro RA,
Duarte Filho J. Efeitos de parâmetros de extrusão nas propriedades físicas de
produtos expandidos de inhame. Ciênc Tecnol Aliment 2006;26(2):459-64.
- Organização das
Nações Unidas para Agricultura e Alimentação – FAO. Bulletin
of statistic 2001. Vol2.
Roma: FAO; 2001.
- Centro Internacional
de Negócios Da Bahia – Promo. Estatística
Internacional e Nacional – exportação e importação de inhame, inhame branco e castanha d’água chinesa. Governo da Bahia,
Secretaria da Indústria, Comércio e Mineração; 2002.
- Lima JA. Potencialidades
de industrialização do inhame e do taro no Brasil. In: Simpósio Nacional sobre
as Culturas do Inhame e do Taro, 2. Joao Pessoa.
Anais. João Pessoa: Emepa/PB; 2002. p. 275-303.
- Heredia Zárate
N.A. Produção de cinco clones de inhame cultivados no pantanal sul-matogrossense. Horticultura Brasileira 1995;13(1):38-40.
- Cereda MP. Justificativa
para a padronização dos nomes vulgares de Cocalasia e
Dioscorea sp. no Brasil. In: Simposio Nacional
Sobre Culturas de Inhame e Taro. Anais. João Pessoa: Emepa/PB;
2002. p.306-7.
- Heredia Zárate
NA, Vieira MC. Composição nutritiva de rizomas em clones de inhame cultivados
em Dourados/MS. Pesquisa Agropecuária Tropical 2004;34(1):61-3.
- Fonseca EWN.
Utilização da mucilagem do inhame (dioscorea spp) como melhorador na produção de pão de forma. 79 p.
março 2006. [Dissertação]. Lavras: Universidade Federal de Lavras/MG; 2006.
- Association of
Official Analytical Chemists International. Official methods of analysis of the Association of
Official Analytical Chemists. 17 ed. Gaithersburg:
AOAC; 2000. v.1.
- Van de Kamer JH, Van Ginkel
L. Rapid determination of crude fiber in cereals. Cereal Chemistry 1952;29(4):239-51.
- Association of Official Analytical Chemists. Official methods of analysis. 15th ed.
Washington: AOAC; 1990. 1298 p.
- Osborne DR, Voogt P. The
analysis of nutrient in foods. London: Academic Press; 1978. p.47, 156-8.
- Cecchi HM. Fundamentos
teóricos e práticos em análise de alimentos. 2 ed. Campinas: Unicamp; 2003.
- Nelson NA. A photometric adaptation of Somogyi method for the determination of glucose. Journal Biological
Chemistry. Baltimore; 1944. p.135-
375.
- Strohecker R, Henning HM. Analises de vitaminas: métodos comprobados. Madrid: Paz Montalvo; 1967. 428p.
- Malavolta E, Vitti
GC, Oliveira SA. Avaliação do estado nutricional das plantas; princípios e
aplicações. 2 ed. Piracicaba: Potafos;
1997. 319p.
- Nagata M, Yamashita I. Simple method for simultaneous determination of
chlorophyll and carotenoids in tomatoes fruit. Nippon Shokuhin
Kogyo Gakkaishi, Tokyo 1992;39(10):925-8.
- Baccou JC, Lambert F, Sauvaire Y. Spectrophotometric
method for the determination of total steroidal sapogenin. Analyst 1977;102(1215):458-65.
- Lees DH, Francis FG. Standardization of pigment
analysis in cranberries. Hortscience 1972;7:83-4.
- Association of Official Analytical Chemists. Oficial methods of analysis. 5a ed. Washington: AOAC; 1960. p.264-265.
- Instituto Adolfo
Lutz. Normas analíticas do Instituto Adolfo Lutz, São Paulo; 1985. V1.
- Ferreira DF. Programa
Sisvar: sistema de análise de variância. Versão 3.04.
Lavras/MG: UFLA; 2000.
- Agencia Nacional de
Vigilância Sanitária. Resolução RDC nº 90, de 18 de outubro de 2000.
Regulamento técnico para fixação de identidade e qualidade de pão. [citado 2007 nov 28]. Available at: http:www.anvisa.gov.br/leisref/public/showact.ph.
- Couto EM. Utilização
da farinha de casca de pequi (Cariocar brasiliense Camb.) na elaboração de pão de forma. [Dissertação].
Lavras: Universidade Federal de Lavras/MG; 2007.
- Heredia Zarate
NA, Vieira MC, Minuzzi A. Produtividade de cinco
clones de inhame custos e uso na panificação caseira. Ciênc
Agrotec 2002;26(6):1236-42.
- Andrade ECB. Analise
de alimentos: uma visão química da nutrição. São Paulo; 2006. 89p.
- El-Dash AA, Camargo
CO, Diaz NM. Fundamentos da tecnologia de panificação. São Paulo: FTPT/PROMOET; sd. 348 p.
- Brillouet JM, Treche S, Sealy L. Alterations in cell wall constituents of
yams dioscorea dumetorum
and D. rontuladata with maturation and storage
conditions, relation with post harvest hardening of
D. dumentorum yam tubers. J Food Sci
1981;46(6):1964-7.
- Ketiku AO, Oyenuga YA. Changes in the carbohydrate constituents of yam tuber
(Dioscorea rotundata pois.) during growth. J Sci Food Agric 1973;24(4):367-73.
- Brasil. Decreto nº
12.486, de 20 de outubro de 1978. Normas técnicas especiais relativas a
alimentos e bebidas. Diário Oficial do Estado de São Paulo, São Paulo, p. 20,
21 out. 1978.
- Leonel M, Cereda MP. Caracterização físico-química de algumas
tuberosas amiláceas. Ciênc Tecnol Aliment 2002;22(1):65-9.
- Agência Nacional de Vigilãncia Sanitária. Legislação específica de alimentos.
Regulamentos técnicos por assunto. Resolução. – RDC n° 90, de 18 de outubro de
2002. [2007 mai 20]. Available at:
http://www.anvisa.gov.br/alimentos/legis/especifica/regutec.
- RDA - National Research Council (USA). Recommended Dietary Allowances. 10 ed.
Washington DC : National Academy Press; 1989. 284p.
- Dietary Reference Intakes (DRI) for Vitamin A, Vitamin K, Arsenic,
Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon,
Vanadium, and Zinc. The
National Academies; 2001.
- Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin
D, and Fluoride (1997); Dietary Reference Intakes for Thiamin, Riboflavin,
Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic
Acid, Biotin, and Choline (1998); Dietary Reference Intakes for Vitamin C,
Vitamin E, Selenium, and Carotenoids (2000).
- Magnoni D, Stefanuto A, Kovacs C. Orientação
nutricional na dislipidemia. IMeN
- Instituto de Metabolismo e Nutrição. 2005. [citado 2007 oct
19]. Available at: http:www.imen.com.br/artigos.
- Schneider E. A cura
pelos alimentos. 1ª ed. Casa Publicadora Brasileira; 1992.
- Ruiz RC, Costa LS,
Silveira M, Brown IF. Seleção de espécies vegetais com potencial de uso, para
estudos ecológicos e manejo, em florestas no oeste da Amazônia. New York: The New York Botanical Garden; 1998. 13 p.
- Staszewski J, Haenszel W. Cancer mortality among the Polish - born in the
United States. J Nat Cancer Inst 1965;35:291-7.
- Olayemi JO, Ajaiyeoba EO. Anti-inflammatory studies of yam (Dioscorea esculenta) extract on wistar rats. African Journal of Biotechnology 2007;6(16):1913-5. [cited 2011 may].
Available at: http://www.ajol.info/index.php/ajb/article/.
- Katalinic V, Milos M, Kulisic T, Jukic M. Screening of
70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem 2006;94:550-7.
- Mélo EA, Lima VLA, Maciel MIS, Caetano ACS, Leal
FLL. Polyphenol, ascorbic acid and total carotenoid
contents in common fruits and vegetables. Braz J Food Technol 2006;9(2):89-94.
- Abramo MA. Taioba. In: Abramo MA. Taioba, cará, inhame: o grande potencial
inexplorado. Ícone; Campinas; 1990. p.57-63.
- Mazza G. Anthocyanins in grape and grape products. Critical Review of Food Science and Nutrition 1995;35:341-71.
- Malacrida C, Motta RS.
Antocianinas em suco de uva: composição e estabilidade. B.CEPPA Curitiba 2006;24(1):59-82.