Rev Bras Fisiol Exerc 2020;19(2):82-94
ORIGINAL
ARTICLE
Aquatic
exercise and cardiac autonomic modulation of postmenopausal women with type 2
diabetes
Exercício aquático e
modulação autonômica cardíaca de mulheres na pós-menopausa com diabetes tipo 2
Eduardo Federighi Baisi Chagas1,2,
Angélica Cristiane da Cruz2, Pedro Henrique Rodrigues2,
Cristiano Sales da Silva2,3, Robison José
Quitério2
1Universidade de Marilia (UNIMAR), Marília, SP,
Brazil
2Universidade Estadual de São Paulo, Campus Rio Claro, Rio Claro/SP, Brazil
3Universidade Federal do Piauí (UFPI), Campus of Parnaíba, PI, Brazil
Received
on: July 31, 2019; accepted on: March 9, 2020.
Corresponding author: Eduardo Federighi Baisi Chagas, Rua
Humaitá, 190 casa 8, 17513-160 Marília SP
Eduardo Federighi Baisi Chagas:
efbchagas@gmail.com
Angélica Cristiane da
Cruz: angelica.cristianedc@hotmail.com
Pedro Henrique
Rodrigues: pedro.edfisica@unimar.br
Cristiano Sales da
Silva: cristiano.silva@ufpi.edu.br
Robison
José Quitério: robison.quiterio@unesp.br
Abstract
Objective: Investigating the effect of 12 weeks of an aquatic exercise program on
cardiac autonomic modulation by heart rate variability index of postmenopausal
women with type 2 diabetes mellitus (T2DM). Methods: A randomized
clinical trial was performed in 25 women aged 51 to 83 years, divided into
exercise group (EG) (n = 13) submitted for 12 weeks to two weekly sessions of
50 minutes each, and control group (CG) (n = 12) without exercise. Results:
Regarding cardiac autonomic modulation significant interaction was observed for
TINN values (ms), indicating a slight increase in EG,
but mostly a reduction in CG. The regression analysis also pointed effect of
aquatic exercise on reducing the LF/HF ratio, after controlling for covariates,
diastolic blood pressure and dyslipidemia. Conclusion: The aquatic
exercise had a significant effect on the reduction of cardiovascular risk,
mainly in relation to glycemia and abdominal obesity, which may represent a
protective effect of exercise in the progression of autonomic dysfunction, but
its effect on autonomic modulation seems to depend on a greater volume and time
with aquatic exercise.
Keywords: diabetes, women, menopause, autonomic nervous system.
Resumo
Objetivo: Investigar o efeito
de 12 semanas de um programa de exercícios aquáticos na modulação autonômica
cardíaca pelo índice de variabilidade da frequência cardíaca (VFC) de mulheres
com diabetes mellitus tipo 2 (DM2) na pós-menopausa. Métodos: Um ensaio
clínico randomizado foi realizado em 25 mulheres com idade entre 51 e 83 anos,
dividido em grupo de exercício (GE) (n = 13), submetido por 12 semanas a duas
sessões semanais de 50 minutos cada, e grupo controle (GC) (n = 12) sem
exercício. Resultados: Em relação à modulação autonômica cardíaca, foi
observada interação significativa para os valores de TINN (ms)
indicando um pequeno aumento no GE, mas principalmente uma redução no GC. A
análise de regressão também apontou o efeito do exercício aquático na redução
da razão LF/HF, após o controle de covariáveis, pressão arterial diastólica e
dislipidemia. Conclusão: O exercício aquático teve um efeito
significativo na redução do risco cardiovascular, principalmente em relação à
glicemia e obesidade abdominal, o que pode representar um efeito protetor do
exercício na progressão da disfunção autonômica, mas seu efeito na modulação
autonômica parece depender de maior volume e tempo com exercícios aquáticos.
Palavras-chave: diabetes, mulheres,
menopausa, sistema nervoso autonômico.
Introduction
The complications in type 2 diabetes mellitus (T2DM) are partly due to
the hyperglycemic state to active toxic pathways in the independent insulin
tissues causing cell damage, which in turn raises the cardiovascular risk [1].
In women the changes of sex steroid hormones in the postmenopausal period is
associated with an increased risk of cardiovascular disease, and
affect the heart rate and the regulation of the autonomic nervous system (ANS)
[2].
In endothelial cells, hyperglycemia alters nerve blood flow decreasing
nerve buffering capacity free radicals, and the depleting energy reserves
available resulting in cellular necrosis and activation of genes involved in
neuronal damage [3]. Thus, chronic hyperglycemia is involved in the destruction
process and the myelin sheath of nerve fibers, resulting in autonomic
dysfunction and decreased heart rate variability (HRV) observed in neuropathies
[4].
The ANS is an important component of the homeostasis control, and the
reduction in capacity has been related to various diseases of the
cardiovascular system [5]. In the case of T2DM, the damage to the ANS is
directly related to the duration of the disease, and therefore, clinical
applications of tools that allow review are of fundamental importance [6]. The
analysis of HRV is one of these methods and allows monitoring of disease
progression and therapeutic efficacy of interventions [7].
As for the treatment strategies in type 2 diabetes, exercise is widely
recommended by contributing to a better glycemic control and reduces
cardiovascular risk factors [8,9]. However the study of the therapeutic effect
of exercise on cardiac autonomic modulation is still recent, and although there
is evidence that aerobic and resistance exercise have a positive effect on the
autonomic modulation in high-risk populations [10] there are also studies that
found no significant improvement after combined aerobic and resistance training
exercise [11,12].
In addition, when considering aquatic exercise, there is little evidence
about the effect of this type of exercise in improving cardiac autonomic
modulation [13,14] mainly in the population of post-menopausal women with type
2 diabetes. Thus, the purpose of the study was to analyze the effect of an
aquatic exercise program on cardiac autonomic modulation by analysis of heart
rate variability in women with postmenopausal type 2 diabetes mellitus.
Methods
Population
of study and casuistic
The sample size (n) was determined to analyze the interaction between
group and intervention time by ANOVA repeated measures between groups. The
sample was considered to calculate an average effect size (0.30), a margin of
error type I (α) of 5% and 80% power study indicating the need for sample
24 sample elements. The calculation of the size of the sample was held at G *
Power software, version 3.1.9.2 (Franz Faul,
University of Kiel, Germany). The sample consisted of 26 women aged 51-83 years
with amenorrhea for at least 12 months, diagnosed with type 2 diabetes for at
least three years and sedentary (<150 minutes per week of moderate or
vigorous exercise in the last three months).
An intervention study was performed (treatment), parallel of two arms,
open-masking and randomized controlled allocation. Figure 1 shows the following
flow chart of the study participants. Patients were submitted to an initial
evaluation with history of the disease, drug therapy, postmenopausal status and
physical activity patterns. After the initial evaluation, the volunteers
included in the study underwent anthropometric averages, fasting blood glucose,
blood pressure and registration of RR intervals (RR intervals) for analysis of
heart rate variability (HRV). Later the volunteers were randomized and
allocated to exercise group (EG) and control group (CG). The allocation was made
through drawing in a sealed envelope. Data collection was performed on two
non-consecutive days and repeated after 12 weeks of the intervention period.
The post-intervention measurements were performed seven days after the end of
the intervention period. After the end of the study the patients allocated to
the CG were invited to participate in aquatic exercise program on the same
terms available to GE.
They were initially included in the study all patients with T2DM and
medical referral to Physical Evaluation Laboratory and Practice of Sports Unimar (LAFIPE-UNIMAR) to practice aquatic exercise.
Patients were not included in the study: they were unable to enter and exit
independently of the pool; inability to understand and follow simple verbal
command; amputations and / or use of prosthetic limbs; stroke sequelae;
Parkinson's disease; fractures of the lower limbs and / or column after 60
years; disabling labyrinth; otitis; hydrophobia; skin lesions; unstable angina;
hypertension uncontrolled and foot deformity. Patients who did not complete the
evaluation protocol and intervention were excluded of the study.
The project was approved by the Ethics Committee of the University of
Marilia-SP (UNIMAR) (n ° 1441220/2016 protocol CAAE: 53040116.2.0000.5496), and
followed the criteria established by resolution of the National Health Council
(CNS 466 / 12). The test was recorded in Rebec (Brazilian Registry of Clinical
Trials) (Registry Number: RBR-8btc25).
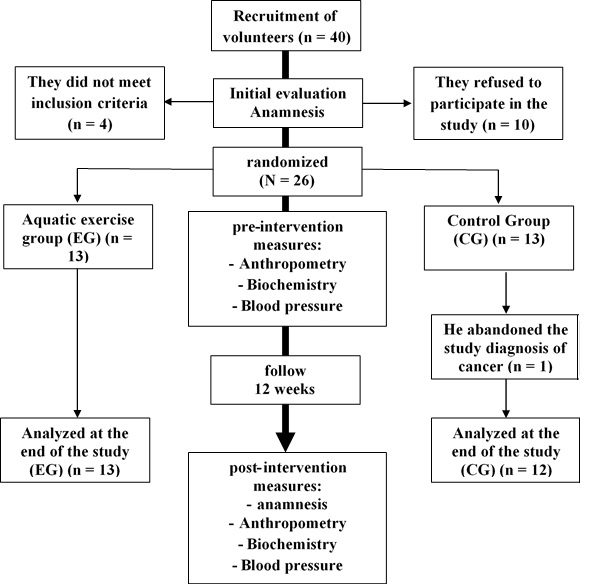
Figure
1 - Flow chart tracking of volunteers
Study
variables
The prevalence of chronic diseases in the study population was obtained
by questionnaire of referred morbidities and confirmed by clinical diagnosis in
the medical this routing. The reported morbidity questionnaire contains
information about the presence or absence of chronic diseases distributed in
metabolic, cardiovascular and rheumatology, as well as the time of diagnosis of
the disease and information on the use of medicines. The questionnaire was
supplemented with information on the pattern of habitual physical activity in
the last three months.
Cardiovascular
risk factors
The fasting blood glucose (FBG) were performed on biochemical analyzer
spectrophotometric reflectance (Accutrend Plus, Roche
Diagnostics, 2007) in venous blood by cubital puncture after overnight fasting
8 hours. Blood pressure (BP) was measured in supine position after twenty
minutes of rest with automatic digital equipment (Omron HEM-742-INT China).
For the analysis of body composition were taken anthropometric
measurements of weight, height and waist circumference (WC). WC values ≥
80 cm were classified as central obesity. The values of the Body Mass Index
(BMI) ≥ 30 were classified as general obesity [15].
Heart
rate variability
Heart rate (HR) and the instantaneous RR intervals were recorded for 20
minutes in the supine position for a digital telemetry system (Polar RS800CX,
Polar Electro Oy, Kempele, Finland). Stable stretches
were selected from 256 points series, and later analyzed in Kubios
Software (HRV version 2.0, University of Kuopio, Finland). In the time domain
the following calculations were made statistical the square root of the average
squared differences between successive regular intervals (RMSSD), expressed in ms; and base width histogram of the RR interval (TINN). For
the analysis in the frequency domain was used the autoregressive method
considering the signal in the bands high-frequency (HF - 0.15 to 0.4 Hz) and
low frequency (LF - 0.04 and 0.15 Hz) for calculating the LF/HF ratio
represents the sympatho-vagal balance. We also
calculated the SD1 and SD2 derivatives of Poincaré
plot [16].
Intervention
procedures
The intervention period was 12 weeks with two weekly sessions lasting 50
minutes each for the exercise group (EG). The control group (CG) received
guidelines for the maintenance of living habits and physical activity
identified in the baseline. The training sessions were held in heated at medium
temperature of 28°C, 1.3 m deep and in groups of up to six volunteers.
In the initial phase (5 minutes) were performed active stretching
exercises lasting 30 seconds each and dynamic exercises in sets of 10
repetitions for the joints, neck, shoulders, elbows, wrists, hips, knee and ankle.
In the main phase (40 minutes) were carried out six pack exercises with 5
minutes each, combining movements of the upper limbs (MMS) and lower limbs
(MMI), totaling 30 minutes [17]. The target intensity was moderate to vigorous,
controlled by the scale of perceived exertion between 12 to 14 points according
to Borg's scale (6 to 20) [18]. The final phase lasted 5 minutes, where
stretching exercises were performed like the initial phase (deltoids, biceps,
triceps, pectorals, dorsal, quadriceps, hamstrings and calf).
Statistical
analysis
Quantitative variables are described as mean and standard deviation
(SD). The qualitative variables are described by the distribution of the
absolute frequency (f) and relative (%). To analyze the association between qualitative
variables was used the Fisher's exact test. The distribution normality was
verified by the Shapiro-Wilk test with Lilliefors correction. To analyze the
effect of intervention between the groups (control vs. exercise) a mixed ANOVA
was built for repeated measures, followed by Bonferroni post-hoc test to
analyze the effect within groups. The effect size was determined by means of
the square values ETA (h2). The delta change (D) (D
= Post training - Pre training) between the pre and post-intervention was used
to quantify the variation of quantitative variables. Multiple linear regression
was used to analyze the effect of the group, as well as the values at baseline
and covariates on the delta change in the HRV index. The R2 was analyzed to
check the determination of the percentage coefficient of variation explained by
the model. For all analyzes, we used the SPSS software version 19.0 for
windows, adopting a significance level of 5%.
Results
The average adherence of the exercise group in aquatic exercise sessions
was 65% for a total of 24 sessions. It was not observed significant differences
between the groups for age, time since diagnosis of T2DM, morbidities and
medication therapy at baseline (Table I). Regarding the use of beta-blockers
(atenolol and propranolol) dosing was observed between 20 to 50 m /day. A
significant reduction in fasting blood glucose, BMI and CC was observed in the
exercise group, but no significant change in systolic (SBP) and diastolic (DBP)
pressure. In the control group there was no significant change in body
composition and blood pressure, except for fasting glucose values and waist
circumference showed a significant increase (Table II).
Table
I - Mean and standard deviation (SD) of age and time
of diagnosis and intervention of the control group
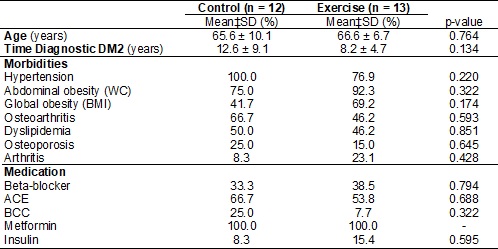
p-value
for average unpaired t-test; for distribution of relative frequency (%) p-value
for Fisher's Exact test. BCC = calcium channel blockers; WC = Waist
circumference; ACE = inhibitors angiotensin converting enzyme; BMI = body mass
index
There was a significant interaction between group and intervention time
for TINN values (ms), indicating improved overall
heart rate variability in EG, and reduction in CG. Although not significant
from the statistical point of view, there was an increase in the values of
RMSSD and SD1 in the EG, and reduction in the CG (Table II). Although no
significant reduction in the EG presented LF/HF ratio, inversely to the behavior
observed in the control group. However, after controlling for DBP and
dyslipidemia it was a significant effect of the intervention with aquatic
exercise on values LF/HF by regression analysis (Table III). The baseline
values showed a significant effect, but discrete, on the variations of values
TINN and SD2. The increase in WC values had a significant effect on the
reduction of TINN values. Although the increase of SBP has shown significant
effect on the increase of RMSSD values and SD1, as well as the increase in BPD
values showed significant effects on the reduction of the LF/HF, these were
discrete and did not demonstrate relevant clinical significance (table III).
Table
II - Mean and standard deviation (SD) of
cardiovascular risk factors and heart rate variability indexes for the control
and intervention groups at the pre- and post-intervention moments
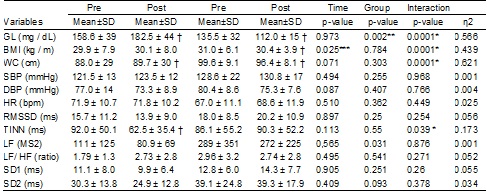
*p≤0,05
significant effect on interaction time vs. grupo;
**significant effect p = 0.05 for differences between groups; ***p ≤ 0,05
significant effect of time. † p = 0.05 significant differences within the group
compared to pre-intervention time by the Post-Hoc test Bonferroni.; eta squared (effect size); WC = Waist circumference;
TC = total cholesterol; HR = heart rate; GL = fasting glucose; BMI = body mass
index; DBP = diastolic blood pressure; SBP = systolic blood pressure; TG =
triglycerides
Table
III - Analysis of the effect of covariates and the
group of the Delta change (D) of the linear rates
of heart rate variability
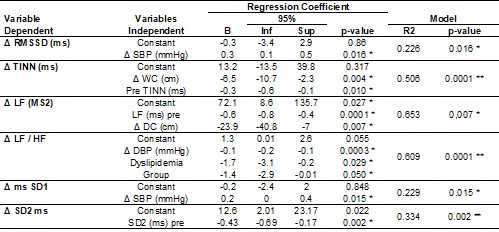
B
= regression coefficient; IC = confidence interval for the regression
coefficient; Inf lower limit; Sup upper limit; *p≤0,05 significant effect
of the independent variable regression coefficient; **p≤0,05 significant
effect of the delta model to predict changes in the dependent variable; R2 =
proportion of variation of the dependent variable explained by the independent
variables; WC
= Waist circumference; Dyslipidemia (0 = absent, 1 = present); Group (0 =
control/intervention = 1); DBP = diastolic blood pressure; SBP = systolic blood
pressure
Discussion
As for the characteristics of the sample was observed high prevalence of
cardiovascular risk factors and osteoarthritis, indicating the effects of both
the aging process associated with post-menopausal condition, as the deleterious
effects of hyperglycemic state related to T2DM [19]. The high prevalence of
comorbid conditions in the sample also suggests that damage to ASN should
already be present, primarily due to the high prevalence of high blood pressure
(hypertension) and time of diagnosis of T2DM in the sample [20]. Although the
use of reference values for interpretation of HRV indices are still of
controversial [21], it can be observed in the present study that the sample
presented in the baseline reduced values of linear indexes representing the
global variability (SD2) and parasympathetic (RMSSD and SD1) [22,23],
suggesting the presence of autonomic dysfunction in the studied sample.
Regarding the effect of intervention with aquatic exercise, we observed
significant reductions in cardiovascular risk factors, but there was no
significant effect of aquatic exercise with intervention on blood pressure and
resting heart rate. Regarding the HRV index, it was a significant interaction
effect only on TINN values (ms) with a slight
increase in EG, but especially significant reduction in CG. Regression analysis
indicated a possible effect of aquatic exercise in reducing the ratio values
LF/HF, but this effect was dependent on the presence of dyslipidemia and
reductions in DBP.
Despite the increase in most linear indices of HRV (RMSSD, SD1 and SD2)
in EG, these were not confirmed by statistical analysis. This is because the
effect of exercise on cardiac autonomic modulation appears to be dependent
overload exercise and the intervention time [24]. The low adherence (65%) of
attendance at training sessions may have influenced the effect of aquatic
exercise on autonomic modulation. However, when considering the high prevalence
of comorbidities in the population of post-menopausal women with type 2
diabetes, poor adherence to exercise programs reflects a clinical reality,
since these patients must be away frequently to appear in medical visits, as
well as to assist in family care [25].
Among the studies that showed significant effect of 12 weeks of physical
exercise of moderate intensity to vigorous on cardiac autonomic modulation, the
use of three weekly sessions proves to be an important aspect [12,26,27], which
indicates that a higher weekly frequency can contribute positively to the
improvement of autonomic modulation. The effect of the increase in weekly
frequency on the extent of improvement in cardiac autonomic modulation can be
observed [28], indicating the importance of this component of the exercise load
on the observable adaptations in cardiac autonomic modulation. But in none of
these studies it was observed using aquatic exercise.
Thus, two weekly sessions may not be enough to observe a significant
effect on cardiac autonomic modulation at 12 weeks of intervention with
exercise of moderate to vigorous, particularly when there is a low adhesion to
training sessions, as found in this study. On the other hand, in the case of a
long-term aquatic exercise intervention [13,14] or on land [11,29], however,
two weekly sessions of moderate to vigorous intensity, significant effects were
observed in the improvement of autonomic cardiac modulation. Thus, the effect
of exercise of moderate to vigorous on autonomic modulation shown dependent on
the relationship between the intervention time and the weekly frequency of
exercise sessions.
Another factor that can influence the effect of intervention with
aquatic exercise in the adaptation of cardiac autonomic modulation response is
the values observed at baseline, which are also related to the health condition
of the patient. It was observed by regression analysis that the reduced values
at baseline were related to higher variations of TINN values and SD2.
Relationship can also be observed in other intervention studies with exercise
[30-32]. The effects of highest amplitude on cardiac autonomic modulation
occurred in those subjects with reduced values at baseline and were associated
with the presence of pathological conditions.
The regression analysis also indicated that factors such as dyslipidemia
and variations in WC, SBP and DBP can also significantly influence HRV
adjustments. Dyslipidemia significant effect on reducing the LF values (nu) and
LF/HF ratio. Although the presence of dyslipidemia and low HRV are related to a
higher cardiovascular risk, the relationship between serum lipids and HRV is
still little studied in patients with diabetes and cardiovascular disease [33].
Nevertheless, the presence of dyslipidemia is an unfavorable condition to
health, and therefore may be related to reduce HRV, which favors its increase
in response to intervention with physical exercise.
Central obesity has been linked to a worse cardiac autonomic modulation
and reduced HRV [10]. It was observed that by regression analysis reductions in
WC contributed significantly increase the overall variability represented by
TINN index (ms). Regarding the effect of reducing the
WC on the reduction of the LF values (ms2), this was observed only
in patients with LF values (ms2) higher at baseline. On the other
hand, for patients with LF values (ms2) reduced the baseline was
observed that reducing the WC contributed to the increase of LF values (ms2).
However, in those patients with LF values (ms2) reduced at baseline,
the increases in their values should be interpreted as a positive adaptation
[30], because very low LF values (ms2) are related to autonomic dysfunction
[31,34].
Although significant effect of varying the PAS on RMSSD values and SD1
by regression analysis, this effect was small, a large variation is required
SBP to produce a considerable effect on these indices of HRV. The reduction PAD
significant effect in reducing the LF/HF ratio, but this effect was more
evident in patients with dyslipidemia. Thus, those volunteers with dyslipidemia
who underwent aquatic exercise and decreased DBP showed the highest reductions
in the ratio values LF/HF. This effect may be related to improved baroreflex
sensitivity, which in turn is related to improvements for the HRV [35,36].
Although 12-week aquatic exercise have produced a slight effect on
cardiac autonomic modulation of postmenopausal women with type 2 diabetes,
decreases in the body composition and FGB parameters indicate a protective
effect of aquatic exercise in the progression of autonomic dysfunction [4,32],
because, from a clinical point of view, the maintenance of cardiac autonomic
balance is directly related to the improvement of glycemic control and
improvement of the health condition of patients with T2DM [37].
A limitation of the study is related to the method used to control the
intensity of physical exercise. Although the use of the Borg effort perception
scale is widely accepted, this isolated method may not be accurate. An
interesting alternative for controlling exercise intensity would be the use of
heart rate monitoring together with the effort perception scale. The use of
heart rate and the stress perception scale in a combined way is important,
because, in the elderly population with diabetes, both autonomic dysfunction
and the use of beta-blocking medication can alter the heart rate response.
Regarding the type of exercise, few studies have examined the
relationship between aquatic exercise and cardiac autonomic modulation [13,14],
and none of them are analyzed in relation population of postmenopausal women
with T2DM. Thus, this study back important contributions to research on the
relationship between water exercise and cardiac autonomic modulation of
patients with T2DM postmenopausal condition. Furthermore, there is evidence
that exercise in water can promote greater adherence to exercise programs,
besides allowing the achievement of exercise vigorously intensity for a longer
time, even in patients with symptoms of pain, osteoarticular diseases and
reduced capacity functional [38,39].
Conclusion
Although the effect of aquatic exercise program on rising TINN values (ms) has been slight and the effect in reducing the LF/HF
ratio was dependent on the reduction of DBP and the presence of dyslipidemia,
factors such as low compliance (65 %) of EG and the low time and number of
weekly sessions seem to explain in part the results. Although the results do
not allow confirmation of a large aquatic exercise effect in the improvement of
cardiac autonomic modulation, the significant reduction of cardiovascular risk
factors indicates that it is the mode of exercise that may at least represent a
protective factor for progression of cardiac autonomic dysfunction in
postmenopausal women with type 2 diabetes.
Declaration
of interest
The authors declare no conflict of interest.
References
- Barbosa JHP, Oliveira
SL, Seara LT. O papel dos produtos finais da glicação avançada (AGEs) no
desencadeamento das complicações vasculares do diabetes. Arq Bras Endocrinol
Metab 2008;52(6):940-50.
https://doi.org/10.1590/S0004-27302008000600005
- Hautamäki H, Mikkola
TS, Sovijärvi ARA, Piirilä
P, Haapalahti P. Menopausal hot flushes do not associate
with changes in heart rate variability in controlled testing: A randomized
trial on hormone therapy. Acta Obstet Gynecol Scand 2013;92(8):902-8.
https://doi.org/10.1111/aogs.12164
- Vinik AI, Maser RE, Mitchell BD, Freeman
R. Diabetic autonomic neuropathy. Diabetes Care [Internet] 2003;26(5):1553-79.
https://doi.org/10.2337/diacare.26.5.1553
- Fleischer
J, Yderstraede K, Gulichsen
E, Jakobsen PE, Lervang HH, Eldrup
E, et al. Cardiovascular autonomic neuropathy is associated with macrovascular
risk factors in type 2 diabetes: New technology used for routine large-scale
screening adds new insight. J Diabetes Sci Technol 2014;8(4):874-80.
https://doi.org/10.1177/1932296814528616
- Lopes PFF, Oliveira
MIB, André SMS, Nascimento DLA, Silva CSS, Rebouças GM, et al. Aplicabilidade
clínica da variabilidade da frequência cardíaca. Rev Neurociencias 2013;21(4):600-3.
https://doi.org/10.4181/RNC.2013.21.870.4p
- Narayanaswamy
N, Moodithaya S, Halahalli
H, Mirajkar AM. Assessment of risk factor for
cardiovascular disease using heart rate variability in postmenopausal women: a
comparative study between urban and rural Indian women. ISRN Cardiol [Internet]. 2013;2013:1-6.
https://doi.org/10.1155/2013/858921
- Stranieri A, Abawajy
J, Kelarev A, Huda S, Chowdhury M, Jelinek HF. An approach
for Ewing test selection to support the clinical assessment of cardiac
autonomic neuropathy. Artif Intell
Med 2013;58(3):185-93. https://doi.org/10.1016/j.artmed.2013.04.007
- Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC et
al. Physical activity/exercise and diabetes: A position statement of the
American Diabetes Association. Diabetes Care
2016;39(11):2065-79. https://doi.org/10.2337/dc16-1728
- American Diabetes Association (ADA). Standards
of medical care in diabetes. Diabetes Care 2019;42(s1).
- Voulgari C, Pagoni
S, Vinik A, Poirier P. Exercise improves cardiac
autonomic function in obesity and diabetes. Metabolism
2013;62(5):609-21. https://doi.org/10.1016/j.metabol.2012.09.005
- Sacre
JW, Jellis CL, Jenkins C, Haluska BA, Baumert M, Coombes JS et al. A six-month exercise intervention in subclinical diabetic heart disease:
Effects on exercise capacity, autonomic and myocardial function. Metabolism
2014;63(9):1104-14. https://doi.org/10.1016/j.metabol.2014.05.007
- Kang
S-J, Ko K-J, Baek U-H. Effects of 12 weeks combined
aerobic and resistance exercise on heart rate variability in type 2 diabetes
mellitus patients. J Phys Ther Sci
2016;28(7):2088-93. Available from: https://www.jstage.jst.go.jp/article/jpts/28/7/28_jpts-2016-178/_article
- Zamunér
AR, Andrade CP, Forti M, Marchi A, Milan J, Avila MA et al. Effects of a hydrotherapy programme on
symbolic and complexity dynamics of heart rate variability and aerobic capacity
in fibromyalgia patients. Clin Exp Rheumatol
2015;33(14):S73-81.
- Albinet CT, Abou-Dest A, André N, Audiffren
M. Executive functions improvement following a 5-month aquaerobics
program in older adults: Role of cardiac vagal control in inhibition
performance. Biol Psychol 2016;115:69-77.
https://doi.org/10.1016/j.biopsycho.2016.01.010
- ABESO. Diretrizes
Brasileiras de obesidade. 4th ed.
https://abeso.org.br/wp-content/uploads/2019/12/Diretrizes-Download-Diretrizes-Brasileiras-de-Obesidade-2016.pdf
- Laborde
S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in
psychophysiological research - Recommendations for experiment planning, data
analysis, and data reporting. Front Psychol 2017;8(2):1-18.
https://doi.org/10.3389/fpsyg.2017.00213
- Olkoski MM, Matheus SC, Moraes
EC, Tusset D. Metodologia para o planejamento de
aulas de hidroginastica. Motricidade 2013;9(3):36-43.
https://doi.org/10.6063/motricidade.9(3).688
- Borg
G. Psychophysical basis of perceived exhertion. Med
Sci Sports Exerc 1982;14(5):377-81.
- Filippatos T, Tsimihodimos
V, Pappa E, Elisaf M.
Pathophysiology of diabetic dyslipidaemia. Curr Vasc Pharmacol
2017;15(6):1-10. https://doi.org/10.5551/jat.RV17023
- Bianchi
L, Volpato S. Muscle dysfunction in type 2 diabetes: a major threat to
patient’s mobility and independence. Acta Diabetol
2016;53(6):879-89. https://doi.org/10.1007/s00592-016-0880-y
- Bauer A, Camm AJ, Cerutti S, Guzik P, Huikuri H, Lombardi F et
al. Reference values of heart rate variability. Heart
Rhythm 2017;14(2):302-3. https://doi.org/10.1016/j.hrthm.2016.12.015
- Lee
CH, Lee JH, Son JW, Kim U, Park JS, Lee J et al. Normative values of short-term
heart rate variability parameters in koreans and their
clinical value for the prediction of mortality. Heart Lung Circ
2018;27(5):576-87. https://doi.org/10.1016/j.hlc.2017.04.009
- Sammito S, Böckelmann
I. New reference values of heart rate variability during ordinary daily
activity. Heart Rhythm 2017;14(2):304-7.
https://doi.org/10.1016/j.hrthm.2016.12.016
- Michael
S, Graham KS, Davis GM. Cardiac autonomic responses during exercise and
post-exercise recovery using heart rate variability and systolic time
intervals-a review. Front Physiol 2017;8:1-19.
- Chagas
EFB, Bonfim MR, Turi BC, Brondino NCM, Monteiro HL. Effect of moderate-intensity
exercise on inflammatory markers among postmenopausal women. J Phys Act Heal
2017;14(6):479-85. https://doi.org/10.1123/jpah.2016-0319
- Sales
ARK, Silva BM, Neves FJ, Rocha NG, Medeiros RF, Castro RRT et al. Diet and
exercise training reduce blood pressure and improve autonomic modulation in
women with prehypertension. Eur J Appl Physiol
2012;112(9):3369-78. https://doi.org/10.1007/s00421-012-2315-y
- Duarte
A, Soares PP, Pescatello L, Farinatti
P. Aerobic training improves vagal reactivation regardless of resting vagal
control. Med Sci Sports Exerc 2015;47(6):1159-67.
https://doi.org/10.1249/MSS.0000000000000532
- Simmonds
MJ, Minahan CL, Serre KR, Gass
GC, Marshall-Gradisnik SM, Haseler
LJ et al. Preliminary findings in the heart rate variability and haemorheology response to varied frequency and duration of
walking in women 65-74 yr with type 2 diabetes. Clin Hemorheol Microcirc
2012;51(2):87-99. https://doi.org/10.3233/CH-2011-1514
- Zoppini G, Cacciatori
V, Gemma ML, Moghetti P, Targher
G, Zamboni C et al. Effect of moderate aerobic exercise on sympatho-vagal
balance in Type 2 diabetic patients. Diabet Med
2007;24(4):370-6. https://doi.org/10.1111/j.1464-5491.2007.02076.x
- Figueroa
A, Baynard T, Fernhall B,
Carhart R, Kanaley JA. Endurance training improves
post-exercise cardiac autonomic modulation in obese women with and without type
2 diabetes. Eur J Appl Physiol 2007;100(4):437-44.
https://doi.org/10.1007/s00421-007-0446-3
- Pagkalos M, Koutlianos
N, Kouidi E, Pagkalos E, Mandroukas K, Deligiannis A.
Heart rate variability modifications following exercise training in type 2
diabetic patients with definite cardiac autonomic neuropathy. Br J Sports Med.
2008;42(1):47-54. https://doi.org/10.1136/bjsm.2007.035303
- Earnest
CP, Poirier P, Carnethon MR, Blair SN, Church TS.
Autonomic function and change in insulin for exercising postmenopausal women. Maturitas 2010;65(3):284-91.
- Badea AR, Nedelcu
L, Valeanu M, Zdrenghea D.
The relationship between serum lipid fractions and heart rate variability in
diabetic patients with statin therapy. Clujul Med
2014;87(3):152.
http://www.clujulmedical.umfcluj.ro/index.php/cjmed/article/view/313
- Dimitropoulos G. Cardiac autonomic
neuropathy in patients with diabetes mellitus. World J Diabetes 2014;5(1):17.
training improves baroreflex sensitivity in type 2 diabetes. Diabetes
2003;52(7):1837-42. https://doi.org/10.2337/diabetes.52.7.1837
- Bernardi L, Bianchi L.
Integrated cardio-respiratory control: insight in diabetes. Curr
Diab Rep 2016;16(11). https://doi.org/10.1007/s11892-016-0804-9
- Jones
SMW, Guthrie KA, LaCroix AZ, Sternfeld B, Landis CA,
Reed SD et al. Is heart rate variability associated with frequency and
intensity of vasomotor symptoms among healthy perimenopausal and postmenopausal
women? Clin Auton Res 2016;26(1):7-13.
https://doi.org/10.1007/s10286-015-0322-x
- Loimaala A, Huikuri
HV, Kööbi T, Rinne M, Nenonen
A, Vuori I. Exercise training improves baroreflex
sensitivity in type 2 diabetes. Diabetes 2003;52:1837-42.
- Igarashi
Y, Nogami Y. The effect of regular aquatic exercise
on blood pressure: A meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2018;25(2):190-9.
https://doi.org/10.1177/2047487317731164
- Rees
JL, Johnson ST, Boulé NG. Aquatic exercise for adults
with type 2 diabetes: a meta-analysis. Acta Diabetol
2017;54(10):895-904. https://doi.org/10.1007/s00592-017-1023-9