Rev Bras Fisiol Exerc 2019;18(3):118-28
ORIGINAL ARTICLE
Caffeine supplementation for 4-day, followed by acute
ingestion, did not impact triathlete output power after submaximal intensity
exercise
A suplementação de cafeína por 4 dias, seguida
de ingestão aguda, não impactou na potência de triatletas após realizarem
exercício de intensidade submáxima
Anderson Pontes
Morales*, Felipe Sampaio-Jorge**, Thiago Barth, D.Sc.***,
Alessandra Alegre de Matos****, Luiz Felipe da Cruz Rangel, M.Sc.*****,
Beatriz Gonçalves Ribeiro, D.Sc.******
*Doutorando
em Ciências Nutricionais pela Universidade Federal do Rio de Janeiro (UFRJ),
Docente dos Institutos Superiores de Ensino do CENSA (ISECENSA), Laboratório de
Pesquisa e Inovação em Ciências do Esporte (LAPICE-UFRJ Macaé/SEMEL),
**Doutorando em Biociências e Produtos Bioativos pela Universidade Federal do
Rio de Janeiro (UFRJ), Docente dos Institutos Superiores de Ensino do CENSA
(ISECENSA), Laboratório de Pesquisa e Inovação em Ciências do Esporte
(LAPICE-UFRJ Macaé/SEMEL), ***Docente da Universidade Federal do Rio de Janeiro
(UFRJ Macaé), Laboratório de Produtos Naturais (UFRJ Macaé), ****Especialista
em Nutrição Clínica Estética pelo Instituto de Pesquisa Ensino Gestão e Saúde
(IPGS), Laboratório de Pesquisa e Inovação em Ciências do Esporte (LAPICE-UFRJ
Macaé/SEMEL), *****Docente da Universidade Estácio de Sá, Laboratório de
Pesquisa e Inovação em Ciências do Esporte (LAPICE-UFRJ Macaé/SEMEL),
******Docente da Universidade Federal do Rio de Janeiro (UFRJ-Macaé),
Laboratório de Pesquisa e Inovação em Ciências do Esporte (LAPICE-UFRJ
Macaé/SEMEL)
Received: September
10, 2019; accepted: September
30; 2019.
Corresponding author: Anderson Pontes Morales, Laboratório de Pesquisa e
Inovação em Ciências do Esporte (LAPICE/SEMEL), Universidade Federal do Rio de
Janeiro - Macaé Campus, RJ, Av. Aluízio da Silva Gomes, Granja dos Cavaleiros,
n. 50, 27930-560 Macaé RJ
Anderson Pontes Morales:
andersonmrl@hotmail.com
Felipe Sampaio-Jorge:
felipesjorge@gmail.com
Thiago
Barth: barththiago@yahoo.com.br
Alessandra Alegre de
Matos: lebuzios@hotmail.com
Luiz Felipe da Cruz
Rangel: luizfeliperangellfr@gmail.com
Beatriz Gonçalves
Ribeiro: ribeirogoncalvesb@gmail.com
Abstract
Introduction:
The aim of this study was to test the hypothesis that caffeine supplementation
(6 mg·kg-1 body mass) for 4-days, followed by acute intake, would impact five
male triathletes output power after performed submaximal intensity exercise. Methods: This was a randomized,
double-blind, placebo-controlled crossover study, placebo (4-day) - placebo
(acute) PP, placebo (4-days) -caffeine (acute) PC, and caffeine (4-day) -
caffeine (acute) CC. Participants abstained from dietary caffeine sources for 4
days and ingested capsules containing either placebo or caffeine (6 mg.kg-1
body mass day in one absorption). The acute trials the capsules containing
placebo or caffeine (6 mg.kg-1 body mass day in one
absorption) were ingested 60min before completing exercise in a treadmill for
40min (80% VO2max) and to perform the Wingate test. Results: Blood lactate was determined
before, 60min after ingestion, and immediately after the exercise on the
treadmill, the Wingate test, and after the recovery (10-min). CC and PC trials
did not change the cardiopulmonary variables (P > 0.05) and the anaerobic
power variables (peak/mean power output and fatigue index) (P > 0.05). The PC
trial compared with PP promoted improvements in the curve power output in 2 sec
by 31.19% (large effect-size d = 1.08; P < 0.05) and 3 sec by 20% (large
effect-size d = 1.19; P < 0.05). A 10min recovery was not sufficient to reduce
blood lactate concentration in the PC trial compared with PP (PC, 13.73 ± 2.66
vs. PP, 10.26 ± 1.60 mmol.L-1; P < 0.05,
respectively) (P < 0.05). Conclusion:
In conclusion, these results indicate that caffeine supplementation (6 mg·kg-1
body mass) for 4 days, followed by acute ingestion, did not impact the
triathletes output power after performed submaximal intensity exercise.
Nutritional interventions may help researchers and athletes to adapt strategies
for manipulating caffeine use.
Keywords: caffeine
metabolism, Wingate test, blood lactate, performance.
Resumo
Objetivo: O objetivo deste
estudo foi testar a hipótese de que a suplementação de cafeína (6 mg·kg-1 de
massa corporal) por 4 dias, seguida de ingestão aguda, afetaria a potência de
cinco triatletas masculinos após realizarem o exercício de intensidade
submáxima. Métodos: Foi realizado um
estudo cruzado, randomizado, duplo-cego, placebo-controlado, placebo (4-dias) -
placebo (agudo) PP, placebo (4-dias) - cafeína (agudo) PC, cafeína (4-dias) -
cafeína (agudo) CC. Os participantes se abstiveram de fontes alimentares de
cafeína por 4 dias e ingeriram cápsulas contendo placebo ou cafeína (6 mg.kg-1
de massa corporal por dia). Os participantes nos ensaios agudos ingeriram
cápsulas contendo placebo ou cafeína (6 mg.kg-1 de massa corporal) 60
minutos antes de realizar o exercício na esteira por 40 minutos (80% VO2máx)
e em seguida realizaram o teste de Wingate. O lactato
sanguíneo foi determinado antes, 60 minutos após a ingestão, imediatamente após
o exercício na esteira e no teste de Wingate e, após
10 min. de recuperação. Resultados:
Nos ensaios CC e PC não foram observadas alterações nas variáveis cardiopulmonares
(P > 0,05) e nas variáveis de potência anaeróbica (potência de pico, potência
média e índice de fadiga) (P > 0,05). No ensaio PC comparado ao PP promoveu
melhorias na curva de potência no tempo de 2 segundos (31,19% - large effect-size d = 1,08;
P < 0,05) e no tempo de 3 segundos (20% - large effect-sized = 1,19; P < 0,05). A recuperação de 10
minutos não foi suficiente para reduzir a concentração de lactato sanguíneo no
ensaio PC em comparação ao PP (PC, 13,73 ± 2,66 vs. PP, 10,26 ± 1,60 mmol.L-1; P < 0,05, respectivamente)
(P < 0,05). Conclusão: Em conclusão,
esses resultados indicam que a suplementação de cafeína (6 mg·kg-1 de massa
corporal) por 4 dias, seguida de ingestão aguda não impactou na potência de
saída de triatletas após realizarem o exercício de intensidade submáxima.
Intervenções nutricionais podem auxiliar pesquisadores e atletas a adaptarem
estratégias na manipulação do uso de cafeína.
Palavras-chave: metabolismo de
cafeína, teste de Wingate, lactato sanguíneo,
performance.
Introduction
Several physiological attributes contribute to the success of endurance
exercises [1], including the interaction between the realization of submaximal
(i.e., ≤ 99% of VO2max) and maximal exercises [2]. To date, a
wide range of acute and chronic interventions have been investigated regarding
performance improvement in endurance exercises [3,4]. Caffeine
(1,3,7-trimethylxanthine) has been described as an effective ergogenic aid for
enhancing performance in various sports [5-10]. Many researchers [11,12] have
argued that the primary focus behind the ventilatory effect (i.e., increased
alveolar ventilation) of caffeine is the central stimulation of the respiratory
medullary complex. The explanations proposed by Chapman and Stager [12] are
that the acute use of caffeine significant increase in minute ventilation (VE)
during submaximal exercise, and this result in a rise in improving arterial
oxyhemoglobin saturation (SaO2) and oxygen delivery to the working musculature.
Furthermore, the acute use of caffeine may improve the coupling
excitation/contraction by facilitating Ca+ exchange of the sarcoplasmic
reticulum and/or by increasing the myofibrillar sensitivity for this ion [9].
This would result in positive changes in the power parameters of athletes
[9,10].
To determine the ideal conditions for maximizing the physiological
effects of caffeine [13], the timing of intake [14] and habituation
[15] have been investigated. Chronic habituation to caffeine impacts the
concentration of A1 and A2A receptors in various brain regions [16,17] and
expression of A2A/A2B receptors distributed in the sarcolemma of skeletal
muscle [18].
This includes A2A expression in the striatum, a subcortical region
essential for coordinating motor activity [19], and oxygen consumption [4].
Moreover, the expression of A2A/A2B receptors of skeletal muscle is involved in
the regulation of contractility of type I and type II fibers [18]. On this
premise, it has been speculated whether caffeine intake exceeding usual
consumption for a shorter period (i.e., four days) could lead to tolerance to
the ergogenic effects of caffeine already demonstrated for long periods (≥
20 days) [4,17].
The aim of this study was to test the hypothesis that caffeine
supplementation (6 mg·kg-1 body mass) for 4-days, followed by acute intake
would impact output power triathletes after performed submaximal intensity
exercise.
Methods
Subjects
Five male triathletes federated from the state of Rio de Janeiro, all
participants in national competitions in Brazil. The athletes presented (mean ±
SD) were aged 33.0 ± 7.4 years, had a body mass of 75.79 ± 8.3 kg, and height
of 179.4 ± 4.3 cm. All of them had at least 3.8 ± 1.2 years of experience in
the sport. No athlete had a medical history of the cardiopulmonary disease or
used any medication during the study. Habitual caffeine consumption was
assessed using an adapted version of the Landrum, Meliska
and Loke [20] caffeine consumption questionnaire. The athletes were regular
consumers of caffeine (242 ± 39 mg.day-1) with < 300 mg.day-1
defined as low habitual caffeine consumption and >300 mg.day-1 as
high habitual caffeine consumption [21]. In addition, a 24-hour dietary record
was completed by each athlete before the first trial, it was then photocopied
and handed back to the athletes so that the same diet could be repeated for
subsequent trials (daily energy, 4016 ± 1119 kcal; carbohydrate, 52.39 ±
17.26%; protein, 16.51 ± 6.68%; fat, 31.08 ± 10.96%). Energy and macronutrient
intake were analyzed by the software Dietpro® 5i (Dietpro, Minas Gerais, Brazil). All participants were
notified of the investigation procedures, requirements, benefits, and risks
before providing written consent. The protocol (2.540.958/2018) was approved by
the Scientific and Ethics Committee of the Federal University of Rio de
Janeiro, Rio de Janeiro, Brazil.
Study design
This is a randomized, placebo-controlled, double-blind crossover study:
placebo (supplementation for 4 days) - placebo (acute supplementation) (PP; n =
5); placebo (supplementation for 4 days) - caffeine (acute supplementation)
(PC; n = 5); and caffeine (supplementation for 4 days) - caffeine (acute
supplementation) (CC; n = 5). Each athlete visited the laboratory on four
occasions. The first visit involved a preliminary confirmation of the maximum
exercise capacity on the treadmill (i.e., cardiopulmonary exercise testing) to
determine maximal oxygen uptake (VO2max) (Table I), assessment of daily
caffeine consumption, familiarization with the protocol in the submaximal
exercise at steady-state treadmill, and familiarization with the test in the
cycle ergometer (Wingate test). From the first to the fourth day, the athletes
were instructed to withdraw all their caffeine consumption (i.e., food sources
of caffeine). The athletes were also instructed to continue the routine per
week training (Table II). Experimental trials involved supplementation for four
days (athletes were instructed to ingest single daily dose at a similar time:
8:00 a.m.) of placebo (250 mg magnesium silicate) or caffeine (6 mg.kg-1
body mass) capsules (first, second, third and fourth days). Acute
supplementation consisted of ingestion of the capsules of placebo (250 mg
magnesium silicate) or caffeine (6 mg.kg-1 body mass; the capsules
were ingested with 250 ml of water) on the day of the experimental trials in
the laboratory (fifth day). During supplementation for four days, the athletes
were monitored by telephone contact, e-mail, and in-person [17]. On the second,
third, and fourth visits (fifth day, acute supplementation), the athletes
arrived at the laboratory (without performing any physical activity 24 h
before) fasting (8 h) and subsequently an intravenous cannula (20G Jelco, B. Braun Medical Inc., USA) was then inserted into a
forearm, and then a blood sample (10 ml) was obtained before capsule ingestion
(Rest). The amount of time to collect each sample was 150 seconds. After ingestion of the capsules, athletes
remained in the tests area for 60 min without performing any physical activity,
and immediately after the second blood sample was collected (AC). The athletes
then underwent a 5-min warm-up on an exercise treadmill (50% VO2max),
and after performing the submaximal exercise in the treadmill for 40 min (80%
VO2max), a blood sample was collected (SE). Then the athletes
performed the test on the cycle ergometer, and two blood samples were collected
immediately after (WT) and 10 min after (R). During the 10-min recovery, the
athletes were lying on a stretcher and did not make any physical effort. Two
days of intervals (i.e., washout) between experimental trials were established.
The experimental trials were performed at the same time of day (7:00 a.m.).
During the experimental trials (fifth day, acute supplementation), the
temperature of the laboratory was regulated between 20°C and 22°C (Figure 1).
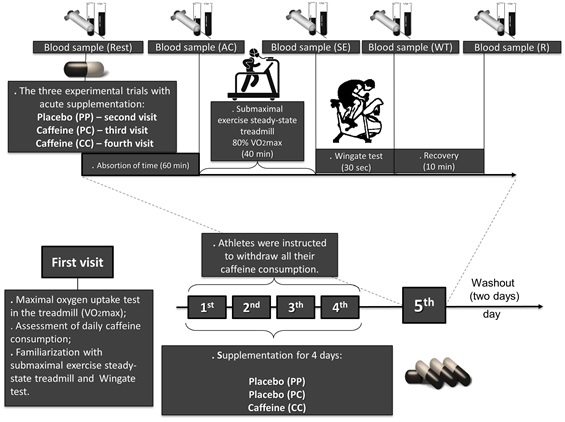
Figure 1 - Experimental design
Maximal oxygen uptake incremental test in the
treadmill
All triathletes were assessed using the same ramp protocol on a
treadmill (ATL/Inbramed, Porto Alegre, Brazil)
programmed to achieve the maximum duration of 8 to 14 min. After a 1-min walk
at 5.5 km/h, the velocity was rapidly increased to 6 km/h and then increased by
0.14 km/h every 7.5 s (1.12 km/h every minute), maintaining treadmill
inclination of 1% [22]. The heart rate was measured every 3 breaths (breath by
breath) from a continuous recording on third derivations (using MC5, V2, and
AVF) measured by a digital electrocardiograph ErgoMet
(HeartWare, Belo Horizonte, Brazil) with the ErgoMet software version 1.0.3.2 (HeartWare).
Ventilatory expired gas was collected with a preVent®
pneumotachograph (MedGraphics, St. Paul, MN) with the
aid of a neoprene mask and was analyzed by VO2000 (MedGraphics),
which was calibrated. The adapted criteria to ensure a maximal exercise test
were (a) achievement of maximum voluntary exhaustion, despite verbal
encouragement, accompanied by a rate of perceived exertion (adapted Borg scale,
0-10 points) and (b) a respiratory exchange ratio greater than 1.10 [22].
Submaximal exercise steady-state treadmill
After the 5-min warm-up on the treadmill (50% VO2max), the
athletes performed the exercise on a steady-state treadmill with a submaximal
protocol of 40 min (80% VO2max). Athletes were monitored every 5min
with the following cardiopulmonary variables: heart rate (HR), maximal oxygen
uptake (VO2), carbon dioxide production (VCO2), minute volume
(VE), O2 expiration fraction (FeO2), and rate of
perceived exertion (RPE) (adapted Borg scale, 0-10 points).
Inertial Wingate test
After performing the submaximal exercise steady-state treadmill, the
triathletes performed the Wingate test. Before the test, the following
instructions were given by the investigators: (i) in
the first seconds of the test, they should pedal from 0 rpm to the greatest
pedaling velocity possible (rpm) for 30 sec; and (ii) maintain this high-power
level during the longest possible time until the test end [23]. Two of the
authors motivated the subjects during the test duration. The Wingate test was
performed in a cycle ergometer of mechanical resistance (Biotec
2100, Cefise®, São Paulo, Brazil) with double sensors
and weights of basket. The resistance was established with each athlete's body
mass (0.075 kg·kg-1). The cycle ergometer was connected to a laptop
using “Ergometric” software (version 7.0, Cefise®)
for the collection and storage of data, such as peak power output (Watts), mean
power output (Watts), fatigue index (peak power output - minimum power output)
/ peak power output × 100) (%) and curve power output (Watts).
Blood caffeine and lactate analysis
The measurement of blood levels of caffeine and lactate was performed in
Rest, AC, SE, WT, and R. Liquid chromatography was adapted from Ribeiro et al. [5]. The liquid chromatographic
analyses were carried out using a Shimadzu chromatograph (Shimadzu® Corp.,
Kyoto, Japan), equipped with an LC-20AT quaternary solvent pump unit, an
SPD-M20A diode array detector operating at 274 nm. The caffeine analyses were
performed at 35°C using a column oven model CTO-20A, and solvent degassing was
performed by a DGU-20A5 degasser. An LCsolution™
software, version 1.25 SP1, was used for system control and data acquisition.
The caffeine was extracted from human serum by a protein precipitation
procedure. Aliquots of 200 µL of drug-free human serum were spiked with 25 µL
of caffeine working solutions or samples obtained from the athletes and were
transferred to 10-mL conical glass tubes. Then, 25 µL of pentoxifylline
solution (60 µg/mL) was used as internal standard and 300 µL of methanol was
added. This mixture was vortex agitated for 1 min and then centrifuged at 3000
rpm for 5 min. Finally, 250 µL of the supernatant was collected, and 50 µL was
analyzed by HPLC. The quantification of caffeine in serum was carried out using
a calibration curve obtained by spiking aliquots of drug-free serum with
working solutions of caffeine at concentrations of 1.2, 3.6, 8, 40, 120, and
240 µg/mL resulting in concentrations of 0.15, 0.45, 1.0, 5, 15, and 30 µg/mL
in a drug-free serum. Plotting was performed by the ratio of caffeine and
internal standard peak areas (y) versus theoretical caffeine concentrations
(x). Measurement of blood levels of lactate was performed to evaluate possible
changes induced by the interventions. After collection, the blood samples were
deposited in tubes with the presence of sodium fluoride. Plasma was obtained by
centrifugation at 2.500 rpm at 4oC for 20 min. The resultant plasma
was stored at -20oC until the analyses could be performed. We used commercial
kits (Labtest, Brazil) and the BIO200 analyzer (Bioplus®, São Paulo, Brazil) [4]. All analyses were made in
triplicate.
Statistical analysis
All values are expressed as mean ± SD and coefficient of variation (CV).
The Shapiro-Wilk test was used to verify the normality of the data. Two-way
analysis of variance (ANOVA) with Tukey post hoc test was used to compare the
differences between the PP, PC, and CC in routine per week training, in blood
measurements (caffeine and lactate), in cardiopulmonary variables (HR, VO2,
VCO2, VE, FeO2 and RPE), and in anaerobic
power (peak power output, mean power output, fatigue index and curve power
output). A Cohen’s d effect size was calculated to quantify the differences
between the PP, PC, and CC trials anaerobic power
(peak power output, mean power output, fatigue index and curve power output)
and concentration lactate. A P value of < 0.05 was considered significant.
Statistical analysis was performed using SPSS 16.0 software for statistical
analyses (SPSS Inc., Chicago, IL, USA).
Ethical approval
All procedures performed in studies involving human participants were in
accordance with the ethical standards of the institutional research committee
and with the 1964 Helsinki declaration and its later amendments or comparable
ethical standards. Informed consent was obtained from all individual
participants included in the study.
Results
The values obtained in the maximum incremental test are shown in Table
I.
Table I - Values obtained in the maximum incremental
test. Mean ± SD and coefficient of variation CV (n = 5)
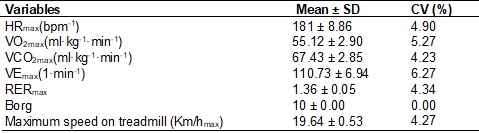
HRmax = maximum heart rate; VO2max
= maximum oxygen uptake; VCO2max = maximum output of carbon dioxide;
VEmax = maximum minute ventilation; RERmax = respiratory exchange ratio; Km/hmax
= maximum speed reached. Adapted Borg rate of
perceived exertion RPE (0-10 points)
In Table II, values obtained in routine per week training prior to acute
experimental trials. There were no significant differences found (P > 0.05).
Table II - Training routine per week (hours). Mean ± SD
(n = 5)
Figures 2 A, B, C, D, E and F show that between both experimental
conditions (PP, PC, and CC), there were no significant differences in
cardiopulmonary responses (group x time interaction: HRP = 0.33; VO2 P = 0.07;
VCO2 P = 0.08; VE P = 0.08; FeO2 P = 0.17) and the rate of
perceived exertion (RPE P = 0.51) over time for 40 min.
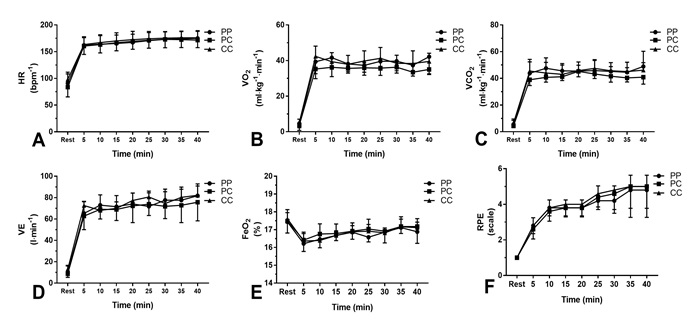
Figure 2 - Cardiopulmonary responses of the following
variables: (A) heart rate, (B) oxygen uptake, (C) output of carbon dioxide, (D)
minute ventilation, (E) expired oxygen fraction, and (F) rate of perceived
exertion. Error bars represent the standard deviation of the mean. (PP,
placebo-placebo [n = 5]; PC, placebo-caffeine [n = 5]; CC, caffeine-caffeine [n
= 5])
Figures 3 A, B, and C shows that between both experimental conditions,
there were no significant differences in peak power output (PC, 826.99 ± 109.57
vs. PP, 779.76 ± 72.44 watts; p = 0.70; CC, 822.84 ± 88.92 vs. PP,779.76 ± 72.44
watts; PC, 826.99 ± 109.57 vs. CC, 822.84 ± 88.92 watts; P = 0.99), mean power
output (PC,645.08 ± 74.91 vs. PP,625.89 ± 47.67 watts; P = 0.98; CC,637.49 ±
67.99 vs. PP,625.89 ± 47.67 watts; P = 0.97; PC, 645.08 ± 74.91 vs.CC, 637.49 ±
67.99watts; P = 0.97) and fatigue index (PC, 68.61 ± 12.85 vs. PP, 75.54 ± 12.88
%; P = 0.57; CC, 79.32 ± 2.98 vs. PP, 75.54 ± 12.88 %; p = 0.84; PC, 68.61 ± 12.85
vs.CC, 79.32 ± 2.98%: large effect-size d = -1.14; P = 0.28). In Figure 3D,
significant differences between the experimental condition in power curve in
time of the 2 sec (PC, 657.05 ± 138.36 vs. PP, 497.54 ± 155.58 watts: large
effect-size d = 1.08; P < 0.05) and 3 sec (PC, 792.48 ± 102.31 vs. PP, 660.00
± 119.45 watts: large effect-size d = 1.19; P < 0.05) were observed.
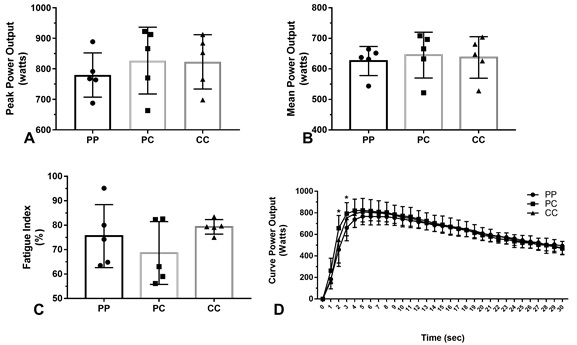
Figure 3 - Power variables in the figures (A) peak power output, (B) mean power output, (C) fatigue index, and (D) curve power output recorded in the Wingate test of the athletes according to the experimental conditions (PP, placebo-placebo [n = 5]; PC, placebo-caffeine [n = 5]; CC, caffeine-caffeine [n = 5]). Error bars represent the standard deviation of the men. (*) Significant difference between the PC vs PP trials (P < 0.05)
In Figure 4, serum concentration of caffeine was not observed in the PP
trial (Rest, AC, SE, WT, and R). We found significant differences (P < 0.05)
in serum caffeine levels in the PC trial (AC, 6.75 ± 0.81 µg/mL-1; SE, 7.29 ± 0.67
µg/mL-1; WT, 7.13 ± 0.57 µg/mL-1; and R, 7.46 ± 1.16 µg/mL-1) and CC trial (AC,
7.19±0.83 µg/mL-1; SE, 7.37 ± 0.674 µg/mL-1; WT, 7.4 7 ± 0.72 µg/mL-1; and R,
7.56 ± 0.85 µg/mL-1) compared to PP trial. In figure 4 B, significant differences
between the experimental condition PC 13.73±2.66 vs. PP 10.26 ± 1.60 mmol.L-1 trials (large effect-size d = 1.58;
P < 0.05) in lactate blood concentration after 10-min recovery (R) were
observed. There were significant differences in the concentration of lactate in
PC trial (Rest 1.11 ± 0.27 vs. ES 5.48 ± 2.71 mmol.L-1; large
effect-size: d = 2.26; P < 0.05) and in CC trial (Rest 1.35 ± 0.55 vs. ES
5.59 ± 3.79 mmol.L-1; large effect-size: d = 1.56; P < 0.05). There
were significant differences in the concentration of lactate in PP (Rest
1.35 ± 0.55 vs. WT 13.52 ± 2.49 mmol.L-1; large effect-size: d = 6.74;
P < 0.05), PC (Rest 1.11 ± 0.27 vs. WT 14.49 ± 2.34 mmol.L-1; large
effect-size: d = 8.03; P < 0.05), and CC (Rest 1.35 ± 0.55 vs. WT 14.33±3.31 mmol.L-1;
large effect-size: d = 5.47; P < 0.05) trials.
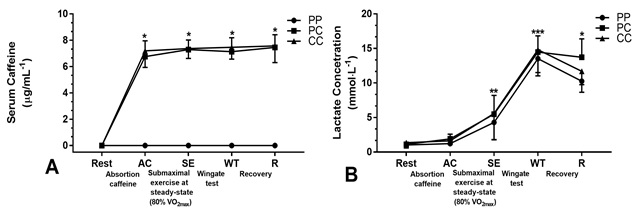
Figure 4 - Analysis of blood samples: (A) serum
caffeine, (B) lactate concentration (PP, placebo-placebo [n = 5]; PC,
placebo-caffeine [n = 5]; CC, caffeine-caffeine [n = 5]). Error bars represent
the standard deviation of the mean. Figure 4A: (*)
Significant difference between the PC, CC vs. PP (P < 0.001). Figure 4B: (*) Significant difference between the PC vs. PP in R
(p < 0.05). (**) Significant difference of PC and CC trials compared to Rest
(P < 0.01). (***) Significant difference of PP, PC, and CC trials compared
with Rest (P < 0.001)
Discussion
The aim of this study was to test the hypothesis that caffeine
supplementation (6 mg·kg-1 body mass) for 4 days, followed by acute intake
would impact output power after performed submaximal intensity exercise. The
present data observed that caffeine supplementation for 4 days, followed by
acute intake did not show changes in cardiopulmonary variables (HR, VO2,
VCO2, VE and FeO2), in RPE and the following anaerobic
power variables: peak/mean power output and fatigue index (P > 0.05). However,
only the PC trial compared with PP trial promoted improvements in the curve
power output in 2 sec by 31.19% (P < 0.05) and 3 sec by 20% (P < 0.05).
Additionally, a 10-min recovery was not enough to reduce blood lactate
concentration in PC trials compared with PP trials (P < 0.05).
Different methodologies have been used by several researchers to explain
the positive ergogenic effects in the use of chronic [15] and acute caffeine
[11]. The influence of a subjects' caffeine habituation is the determining factor
in the verification of an ergogenic response, which is often neglected in many
studies [24,13,14], although evidence shows that this interferes with physical
performance after acute supplementation [15]. To minimize this conflict, all
triathletes were classified as low caffeine users (242 ± 39 mg·day-1)
[18].
A recent positron emission tomography (PET) study Elmenhorst
et al. [25] showed that almost half of the brain A1receptors were occupied by
caffeine use when participants received an intravenous dose of 4.3 mg·kg-1 body
mass, which corresponded to a plasma concentration of ~8 µg/mL-1. We used a
dosage of 6 mg.kg-1 caffeine body mass and observed a peak serum
concentration of (PC) 7.46 ± 1.16 and (CC) 7.56 ± 0.85 µg/mL-1) (Figure 4A) similar to the
study (6.59 ± 4.44 µg/mL-1) conducted by our research group, resulting in
increased athletic performance [5]. This dose represents a posology of 450 mg
of caffeine for 75 kg body mass (equivalent of 5 expresso cup), exceeding the
usual consumption of the triathletes studied (242 ± 39 mg·day-1).
There is evidence that this dosage (6 mg·kg-1 body mass) may be capable of
promoting reduction in the rate of perceived exertion [10]. However, we did not
observe changes in RPE in PC and CC trials (Figure 2 F, P > 0.05) even under a
high blood lactate concentration observed after submaximal exercise in relation
to Rest (Figure 4 B, P < 0.01).
It is known that the rate of absorption may vary among athletes [5], and
the hypothesis that 4 days habituation to caffeine could influence the increase
in absorption rate was questioned [17]. However, we did not observe differences
between PC and CC trials (Figure 4 A, P > 0.05). The explanations are that
this could facilitate the metabolization and increase the concentrations of paraxanthine,
theobromine, and theophylline (caffeine metabolites not verified in the present
study) [26-28]. In view of these arguments, we believe that chronic use of
caffeine in the plasma is an important step in not inducing a positive
ergogenic effect. Therefore, it was expected that only the acute use of
caffeine (PC) could promote improvements in performance in endurance during the
submaximal exercise on the treadmill [3]. However, we did not verify these
changes by cardiopulmonary responses (Figures 2 A, B, C and D, P > 0.05). The
findings of several studies show that the main mechanism that undergoes the
influence of caffeine use is the respiratory medullary complex [27,10,11]. It
is known that elevated VE may increase the partial pressure of alveolar/muscular
oxygen and consequently induce improvements in performance in endurance [11].
In certain investigations, they failed to observe these findings, because the
acute use of caffeine during the performance of a submaximal exercise at steady
state (i.e., above 75% the VO2max) revealed increases in diaphragm
muscle fatigue [29,11]. Therefore, it does not seem prudent that fatigue of the
diaphragm muscle could be associated to the findings observed in our study,
because the fatigue installed in this muscle can promote significant reductions
in the VE [11], and this was not observed (Figure 2 D, P > 0.05). We believe
that the intensity (80% VO2max) of the submaximal exercise protocol
in the present study was high about the cardiopulmonary condition of the
athletes studied (Table I) to observe changes in cardiopulmonary parameters
with caffeine use.
It is postulated that as a result of a resensitization
of adenosine receptors (A2A/A2B) in skeletal muscle induced by the withdrawal
to the chronic use of caffeine, may have induced an improvement in performance
[28,17]. However, Irwin et al. [15]
showed similar improvements in time-trial exercise with caffeine in habitual
consumers regardless of a 4-day withdrawal period. Similarly, Van Soeren & Graham [24] showed equal improvements in time
of exhaustion with acute caffeine supplementation in habituated consumers
following no, 2-days and 4-day of caffeine withdrawal. Although our findings
indicate that the acute use of caffeine (PC) improved the performance (Figure 3
D, P < 0.05) and exhibition high values of lactate in recovery (PC 13.73 ± 2.66
vs. PP 10.26 ± 1.60 mmol·L-1P < 0.05 Figure 4 B), the 4 days period use of
caffeine (CC) did not impact the mean/peak power output and fatigue index
compared to placebo (PP) (Figure 3 A, B, and C P > 0.05). However, the results
observed individually of the 5 triathletes, PC; 4 and PC/CC; 3 triathletes
responded to the use of caffeine (peak/mean power output) (Figure 3 A-B).
These results should be interpreted with caution considering
inter-individual variability observed in the metabolism of caffeine. According
to Ribeiro et al. [8], genetic polymorphisms in related genes to caffeine
metabolism (aryl-hydrocarbon receptor [AHR], cytochrome P450 1A1 and 1A2
(CYP1A1-CYP1A2, Prenyl (Decaprenyl)) are a potential
explanation for the variability in the ergogenic response to caffeine
supplementation in trained athletes. Given these prior findings, it could be
hypothesized that a slower metabolism would be advantageous for maximizing the
ergogenic benefit of caffeine [28]. The limitation of this study is that we
were unable to evaluate the polymorphisms in related genes to caffeine
metabolism. This could minimize the impact on interindividual variability of a
small sample of athletes and favor an improvement in statistical testing power.
Other studies [4,15,17] questioned that changes in athletes' training
routines could induce doubtful findings regarding the effects of caffeine use.
However, we did not observe significant changes in training routine per week
(Table II, P > 0.05). This suggests that triathletes have maintained similar
physical capacity over the study period. Therefore, any influence on
performance during the performance of the Wingate test in any experimental
trial (PP, PC, and CC) is due to the 4 days period and acute supplementation.
Conclusion
In conclusion, these results indicate that caffeine supplementation (6
mg·kg-1 body mass) for 4-day, followed by acute ingestion, did not impact the
triathletes output power after performed submaximal intensity exercise. The
intensity (80% VO2max) of the submaximal exercise protocol in the
present study was high about the cardiopulmonary condition of the triathletes
studied to observe changes in cardiopulmonary and output power parameters using
the recommended caffeine dose. Thus, nutritional interventions may help
athletes to adapt strategies for manipulating caffeine use. Sports and
activities that alternate aerobic and anaerobic power would be applicable to
the results of this study.
Acknowledgements
The authors thank the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), the
triathletes of the cities of Macaé/RJ and Campos dos Goytacazes/RJ, the professor Henrique Mota
for the collections of blood, Paula Pontes and Alessandra Neves for the
translation and the scientific initiation students of our laboratory. This study was financed in part by the Coordenação de Aperfeiçoamento de pessoal de Nível Superior - Brasil (CAPES) (Finance Code 001).
References
- Bentley
DJ. Millet GP,Vleck VE,
McNaughton LR. Specific aspects of contemporary triathlon: implications for
physiological analysis and performance. Sports Med 2002;32(6):345-9.
https://doi.org//10.2165/00007256-200232060-00001
- Bonsignore MR, Morici G, Abate P, Romano S, Bonsignore
G. Ventilation and entrainment of breathing during cycling and running in triathletes.
Med Sci Sports Exercise 1998;30(2):239-5.
https://doi.org/10.1097/00005768-199802000-00011
- Barnes
KR, Kilding AE. Strategies to improve running
economy. Sports Med 2015;45(1):37-56. https://doi.org/10.1007/s40279-014-0246-y
- Lara
B, Ruiz-Moreno C, Salinero JJ, Del Coso J. Time course of tolerance to the performance
benefits of caffeine. PLoS One
2019;14:e0210275. https://doi.org/10.1371/journal.pone.0210275
- Ribeiro BG, Morales AP,
Sampaio-Jorge F, Barth T, de Oliveira MB, Coelho GM et al. Caffeine attenuates decreases in leg power without increased muscle
damage. Journal of Strength & Conditioning Research 2016;30(8):2354-2360.
https://doi.org/10.1519/JSC.0000000000001332
- Powers
SK, Dodd S, Woodyard J, Magnum M. Caffeine alters ventilator and gas exchange
kinetics during exercise. Med Sci Sports Exercise 1986;18(1):101-6.
- Assi HN, Bottoms L.The effects of caffeine on rugby passing accuracy
while performing the Reactive Agility Test. Sci Sports 2014;29(5):275-81.
https://doi.org/10.1016/j.scispo.2014.07.012
- Ribeiro
BG, Morales AP, Sampaio-Jorge F, Tinoco FS, MatosAA, Leite TC.Acute
effects of caffeine intake on athletic performance: A systematic review and
meta-analysis. Revista Chilena
de Nutrición 2017;44(3):283-91.
https://doi.org/10.4067/s0717-75182017000300283
- Wilk
M, Krzysztofik M, Maszczyk
A, Chycki J, Zajac A. The acute effects of caffeine
intake on time under tension and power generated during the bench press
movement. International Society of Sports Nutrition 2019;16(1):8.
https://doi.org/10.1186/s12970-019-0275-x
- Grgic J, Mikulic
P, Schoenfeld BJ, Bishop DJ, Pedisic Z. The influence
of caffeine supplementation on resistance exercise: A review. Sports Med
2019;49(1):17-30. https://doi.org/10.1007/s40279-018-0997-y
- Chapman
RF, Mickleborough TD. The effects of caffeine on
ventilation and pulmonary function during exercise: an often-overlooked
response. Physician and Sports Medicine 2009;37(4):97-103.
https://doi.org/10.3810/psm.2009.12.1747
- Chapman
RF, Stager JM. Caffeine stimulates ventilation in athletes with
exercise-induced hypoxemia. Medicine & Science in Sports & Exercise
2008;40(6):1080-6. https://doi.org/10.1249/MSS.0b013e3181667421
- Hodgson
AB, Randell RK, Jeukendrup AE. The metabolic and
performance effects of caffeine compared to coffee during endurance exercise. PLoS One 2013;8(4):e59561.
https://doi.org/10.1371/journal.pone.0059561
- Skinner
TL, Jenkins DG, Taaffe DR, Leveritt MD, Coombes JS.
Coinciding exercise with peak serum caffeine does not improve cycling
performance. J Sci Med Sport 2013;16(1):54-9.
https://doi.org/10.1016/j.jsams.2012.04.004
- Irwin
C, Desbrow B, Ellis A, Rant BO, Leveritt
M. Caffeine withdrawal and high-intensity endurance cycling performance. J
Sports Sci 2011;29(5):509-15. https://doi.org/10.1080/02640414.2010.541480
- Johansson
B, Ahlberg S, van der Ploeg I, Brené
S, Lindefors N, Persson H et al. Effect of long term caffeine treatment on A1 and A2 adenosine receptor
binding and on mRNA levels in rat brain. Naunyn-Schmiedeberg's
Archives of Pharmacology 1993;347(4):407-14. https://doi.org/10.1007/bf00165391
- Beaumont
R, Cordery P, Funnell M, Mears S, James L, Watson P.
Chronic ingestion of a low dose of caffeine induces tolerance to the
performance benefits of caffeine. J Sports Sci 2017;35(19):1920-27.
https://doi.org/10.1080/02640414.2016.1241421
- Lynge J, Hellsten
Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal
muscle. Acta Physiologica Scandinavica
2000;169(4):283-90. https://doi.org/10.1046/j.1365-201x.2000.00742.x
- Yang
JN, Chen JF, Fredholm BB. Physiological roles of A1
and A2A adenosine receptors in regulating heart rate, body temperature, and
locomotion as revealed using knockout mice and caffeine. Am J Physiol Heart Cir Physiol 2009;296(4):H1141-1149.
https://doi.org/10.1152/ajpheart.00754.2008
- Landrum RE, Meliska CJ, Loke WH. Effects of caffeine and task experience on task performance. Psychologia: An International Journal of Psychology in the
Orient 1988;31(2):91-7.
- Westerterp-Plantenga MS, Lejeune MP,
Kovacs EM. Body weight loss and weight maintenance in relation to habitual
caffeine intake and green tea supplementation. Obes Res
2005;13(7):1195-1204. https://doi.org/10.1038/oby.2005.142
- Morales AP,
Sampaio-Jorge F, Cruz Rangel LF, Souza MJ, Leite TC, Ribeiro BG. Cardiopulmonary performance during maximal exercise in soccer players
with alterations in renal function. J Hum Kinet
2017;57(1):107-15. https://doi.org/10.1515/hukin-2017-0052
- Bar-Or
O. The Wingate anaerobic test. An update on methodology, reliability and
validity. Sports Med 1987;4(6):381-94.
https://doi.org/10.2165/00007256-198704060-00001
- Van
Soeren MH, Graham TE. Effect of caffeine on
metabolism, exercise endurance, and catecholamine responses after withdrawal. J
Applied Physiol 1998;85(4):1493-501.
https://doi.org/10.1152/jappl.1998.85.4.1493
- Elmenhorst D, Meyer PT, Matusch A, Winz OH, Bauer A.
Caffeine occupancy of human cerebral A1 adenosine receptors: in vivo
quantification with 18F-CPFPX and PET. Journal of Nuclear Medicine
2012;53(11):1723-29. https://doi.org/10.2967/jnumed.112.105114
- Svenningsson P, Nomikos
GG, Fredholm BB. The stimulatory action and the
development of tolerance to caffeine is associated with alterations in gene
expression in specific brain regions. J Neurosci
1999;19(10):4011-22.
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that
contribute to its widespread use. Pharmacological Reviews 1999;51(1):83-133.
- Gonçalves
LS, Painelli VS, Yamaguchi G, Oliveira LF, Saunders
B, Silva RP, et al. Dispelling the myth that habitual caffeine consumption
influences the performance response to acute caffeine supplementation. J
Applied Phys 2017;123(1):213-20. https://doi.org/10.1152/japplphysiol.00260.2017
- Mayock
DE, Standaert TA, Woodrum DE. Effect of
methylxanthines on diaphragmatic fatigue in the piglet. Pediatr Res 1992;32(5):580-4.
https://doi.org/10.1203/00006450-199211000-00019