Rev Bras Fisiol Exerc 2020;19(2): 124-33
ORIGINAL
ARTICLE
Association
between frailty and peripheral arterial disease
Associação entre
fragilidade e doença arterial periférica
Sergio Ribeiro
Barbosa¹, Natália Rodrigues dos Reis², Henrique Novais Mansur³
1Faculdade de São Lourenço, Departamento de Educação Física, São Lourenço, MG, Brazil
2Universidade Federal de Juiz de Fora, MG, Brazil
3Instituto
Federal do Sudeste de Minas Gerais, Departamento Acadêmico de
Educação (DAE), Núcleo de Educação
Física e Saúde, Rio Pomba, MG, Brazil
Received
on: January 7th, 2020; Accepted on: April, 16th, 2020
Corresponding author: Natália Rodrigues dos
Reis, Avenue José de Assis Vieira Journalist,
19 Jardim América Rio Pomba MG, Brazil
Sergio Ribeiro Barbosa:
sergior.barbosa@yahoo.com.br
Natália Rodrigues dos
Reis: natyrreis@hotmail.com
Henrique Novais Mansur:
henrique.mansur@ifsudestemg.edu.br
Abstract
Background: Frailty has been associated with lifestyle, chronic diseases, and
genetic alterations and with high levels of proinflammatory proteins,
justifying the relationship proposed by the emerging literature that seeks
associations between frailty and cardiovascular diseases. Objective: To
investigate clinical and sociodemographic factors associated with frailty,
emphasizing the relationship with peripheral arterial disease. Methods:
Medical records of 76 patients were analyzed, considering the results of the
ankle-brachial index test, fragility, sociodemographic and clinical variables.
After the descriptive analysis, differences between groups were tested by
chi-square test, student’s t-test and Tukey's post hoc test, when they were
appropriated. The value of p < 0.05 for statistically significant
differences was considered. Results: The prevalence of frailty in the
study sample was 22.3%, and 47.3% for pre-frail. Frailty was associated with
female gender, hypertension, dyslipidemia and level of education. Changes in
ankle-brachial index test were statistically associated with frailty. Conclusion:
The results of this research show the necessary targeted efforts to prevent and
treat frailty.
Keywords: peripheral arterial disease; cardiovascular disease; chronic disease;
fragility.
Resumo
Introdução: Fragilidade tem sido
associada com hábitos de vida, doenças crônicas, alterações genéticas e níveis
elevados de proteínas pró-inflamatórias, justificando a emergente relação
proposta entre Fragilidade e doenças cardiovasculares. Objetivo:
Investigar fatores clínicos e sociodemográficos associados à Fragilidade,
enfatizando-se a relação com a doença arterial periférica. Métodos:
Foram analisados prontuários de 76 pacientes, considerando-se valores do Índice
Tornozelo-Braquial (ITB), Fragilidade, variáveis sociodemográficas e clínicas.
Após análise descritiva, testou-se as diferenças pelo teste Qui-quadrado,
t de Student e Post Hoc de Tukey,
quando apropriado. Considerou-se p < 0,05 para diferenças significativas. Resultados:
A prevalência de frágeis foi de 22,3% e de pré-frágeis
47,3%. Fragilidade associou-se ao sexo feminino, hipertensão arterial,
dislipidemia e ao analfabetismo funcional. Alterações no ITB foram
estatisticamente relacionadas à Fragilidade. Conclusão: Os resultados
dessa pesquisa alertam para necessidade de esforços direcionados a intervenções
para prevenção e tratamento adequado da Fragilidade.
Palavras-chave: doença arterial
periférica; doenças cardiovasculares; doenças crônicas; fragilidade.
Introduction
The peripheral arterial disease (PAD) comprises a distinct group of
diseases and syndromes, which affect the arterial, venous and lymphatic
systems. It is featured by stenosis, aortic occlusion or limb arterial
occlusion that affects the regular flow of blood or lymph [1]. Prognosis is
related to the mortality increase, ulcers, amputation risk, deterioration of
function capacity and life quality. It is also an independent strong predictor
for arterial coronary and cardiovascular disease.
PAD or other comorbidities presence may enhance the appearance of
geriatric complications, such as Fragility, which currently is an important
challenge for public health [3]. Fragility, based on sarcopenia, neuroendocrine
deregulation and immune dysfunction, presents the functional reservation loss
and the capacity diminish to respond to stressful stimuli, which is considered
the state between the health ideal and the decline for dependence and death
[4]. However, besides the problems related to senescence, researches point the
relation between fragility with lifestyle, chronic diseases, genetic
alterations and with high levels of proinflammatory proteins [5], justifying
the proposed relation by the emerging literature that searches association
between fragility and cardiovascular diseases [6].
The high prevalence of cardiovascular disease and the adverse outcome of
fragility confirm the necessity to improve the associative knowledge among the
factors, contributing to elaborate strategies in order to prevent negative
outcomes, such as falls, hospitalizations and deaths [4].
By the afore mentioned, the objective of the present study was to
investigate clinic and sociodemographic factors associated to fragility,
emphasizing the relation with PAD.
Methods
The current study was performed at Nephrology Treatment, Research, Study
Interdisciplinary Center of Federal University of Juiz de Fora, state of Minas
Gerais, Brazil (NIEPEN).
It is a descriptive survey of cross-sectional cohort, sample composed by
76 individuals from both genders with average age 64.17 + 11.2 years.
Inclusive
criteria
For the research, patients from Hiperdia Minas
Center of secondary health attention were considered, which according to the
analysis of medical record, have been assessed for fragility and ankle-brachial
index (ABI), with a maximum interval of six months for more
or less between performing both.
Criteria for conducting the ABI were those adopted by the unit where the
patients are cared. These patients aged over 65 years old and/or diabetic,
hypertensive, smokers, with family history of PAD, complaints of intermittent
claudication, diminish of peripheral arterial pulse, skin trophic alterations,
dyslipidemia, hyper-homocysteinemia or presence of
inflammatory markers [7]. The fragility evaluation is part of physical
education care protocol, in which patients are selected according to the daily
demand.
Patients who did not perform the fragility evaluation and the
ankle-brachial index were not included in the research. If the patient
performed only one test or did not complete it, their participation was
excluded from the study sample.
If the patient did not conclude the fragility evaluation or did it
partially, it happened due to medical contraindications, which are:
amputations, extreme physical sequelae of stroke, Parkinson disease, pregnancy,
advanced stage of neoplasias and infection by Human Immunodeciency Virus (HIV)
Clinical
and sociodemographic data
Clinical and sociodemographic data were collected through the patient
medical record analysis, and the following parameters were selected: gender,
race (self reporting), level of education, smoking
and alcoholism, systemic arterial hypertension, diabetes, chronic kidney
disease, dyslipidemia, obesity (Body Mass Index), left ventricular hypertrophy,
angina/acute myocardial attack, myocardial revascularization/angioplasty,
transient ischemic attack, coronary disease, retinopathy and heart
insufficiency.
Fragility
To determine frailty, Fried et al. [4]
proposal was adopted. This is a construct based on the evaluation of hand grip
strength, weight loss > 5kg, nonintentional, in the
previous year; fatigue; walking speed and physical activity level. Frail
individuals are considered when they score 3 or more evaluated criteria,
pre-frail individuals when they score 1 ou 2 points
and non-frail the ones who did not score.
Peripheral
arterial disease
To check the PAD presence, the Ankle-Brachial Index (ABI) was applied.
For that, the systolic blood pressure measured was divided at the tibial region
by the systolic blood pressure measured at the humeral region [8].
To interpret data, the most affected limb was considered. Values lower
than 0,90 refer to peripheral flow obstruction and higher than 1,30 indicate
arterial stiffness. Results between 0,90 and 1,30 are considered as PAD absence
perceived by the test and are pondered as regular ones.
Statistical
analysis
Statistical Package for the Social Sciences (SPSS) 20.0 program was used
to interpret data. To data extrapolatory analysis of the sample in general and
of the Frailty categories, descriptive statistics was used such as frequency,
mean and standard deviation, when they are appropriate. Differences between
groups were tested in proportion cases by chi-square test, and in case of
continuous variables, by the student’s t-test, followed by Tukey’s Post Hoc
test. The value of p<0.05 was considered for the significance test among the
variables and groups.
Ethical
aspects
All the ethical principles were respected, according to 196/96
Resolution of National Health Council. The present study was previously
approved by Humam Being Reasearch
Ethics Committee of Santa Casa de Misericórdia in
Juiz de Fora, MG under the technical opinion 566.668 (CAAE
25682813.4.0000.5139).
Results
Table I presents clinical and sociodemographic descriptive results
typical of the studied total sample and its division among the Frailty groups
Table
I - Clinical, laboratory and sociodemographic data
divided in frail, pre-frail and non-frail groups
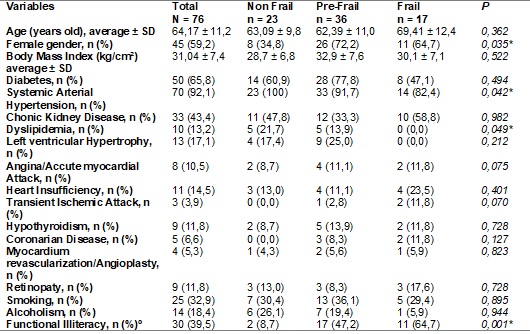
*Significative
difference p<0,05. º education level lower to 4 years
Frailty high prevalence found (22.3%) is statistically significative and
connected to female gender, systemic arterial hypertension, dyslipidemia and to
education level.
Results of frailty analysis are shown at table II by the ABI test
results. Data present statistical signifivative
difference among the groups. From the individuals with ABI alteration, 32.3%
were frail and 51.6% were pre-frail.
Table
II - Relation between fragility and ABI test results

*Significative
difference p < 0.05
The relation among fragility criteria and ABI are found at Table III.
Three out of five criteria evaluated for fragility are checked - physical
education level, hand grip strenght and walking speed
- individuals with ABI altered scored more than patients without alteration.
For the exhaustion report and non-intentional weight loss variables, no
significative difference was found, however, the high prevalence is remarkable
in both groups.
Table
III - Relation among the fragility criteria and ABI
test results
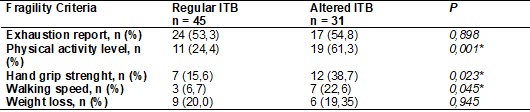
*Significative
difference p < 0,05
Discussion
The objective of the present study was to investigate clinical and
sociodemographic factors associated to frailty, emphasizing the relation with
peripheral arterial disease.
From the 76 individuals assessed, 22.3% were considered frail. Such
values are expressively higher than the national average, which has been
pointed in studies that follow the same fragility evaluation protocol, as
demonstrated by Duarte et al. [9] who evaluated 1399 elderly of SABE
study in São Paulo city and found a prevalence of frail elderly of 8.5%. When
compared to international studies, the results are still relevant because they
are superior than the average found by Manfredi et al. [10], which was
7.7% when 60816 elderly who live in European countries were assessed. And when
it comes to North American studies, whose percentage was 6.9%, the average is
also superior, verified by Fried et al. [4].
We also found 47.3% of the evaluated as pre-frail. Our results are
superior than what was found by a Brazilian multicenter study [9], whose
average was 41.5% and by an European multicenter
study, which found a prevalence of 42.9% of pre-frail [10]. However, these data
become more pertinent due to the dynamic features of Fragility. A pre-frail
individual presents enough physiological reservations to properly respond to
some stressing events, but the fragility presents silent features and may rapidly
get worse to a frail framework and its associated complications [11], and in
contrast, it may positively respond to the treatment and evolve to a non-frail
classification. Trevisan et al. [12] showed
the syndrome negative evolution in a study evaluating 2,925 Italian elderly.
The transition from the robust to pre-frail and from the pre-frail to frail
happened with 954 individuals and 745 died. The individuals were revaluated
after 4.4 years and the mortality rate was 2.4 times higher in frail individuals
[12]. Duarte et al. [9] found a syndrome negative evolution, being 11.8%
coming to death, 0.3% being institutionalised and
39.8% higher in frail individuals.
Responding to the initial objective of our study, it was verified that
from the frail total sample, the individuals with altered ABI, 32.3% were
classified as frail and 51.6% as pre-frail. These values are substantial even
when compared to studies which analyzed Fragility associated with other
clinical conditions. For example, Xue et al.
[13] found a prevalence of 21% of Fragility in 171 patients hospitalized and
reported that atherosclerosis was a risk factor for fragility. At the same
study [9], ABI presented lower indexes in frail patients than in pre-frail and
non-frail, suggesting a correlation between Fragility and cardiovascular
disease.
Although physiopathology of cardiovascular disease and Fragility are
complicated, both present common biological paths, which might explain the
association, found among the variables.
Inflammatory state, reflected by rolling inflammatory markers, such as
interleukin-6 and C-reactive protein, as well as thrombotic markers (VIII e
D-dimers) bring strong correlation between the disease and frailty. Systemic
inflammation induces to the activation of atherosclerotic plaques in the
cardiovascular disease through oxidation of lipoproteins, being associated with
arterial rigidity and promoting a muscle catabolic neuro-hormone state [14],
essential component at the frailty circle. Besides, it permeates a boosting
imbalance of osteopenia, anorexia, immune and cognitive decline, hematological
and metabolic problems [6], which are also associated to Fragility. Reinforcing
such relation, it was already proved that frail inflammatory individuals
present up to 16% more chances to develop early frailty when compared to
non-inflammatory individuals.
Another feasible association between Fragility and PAD is by oxidative
stress. It is defined as an imbalance between formation and removal of
oxidative agents in the organism, and has been related to vascular
pathogenesis, linked to arterial stiffness and to arterial lumen reduction
[16]. In a cellular level, the oxidative stress has also been postulated as a
feasible mechanism that leads to Fragility. Due to cellular division
alterations and to protein production associated to pathological processes
and/or aging, there is a telomere loss. Such loss, brokered by oxidative
imbalance, is strongly linked to a physiological decline in elder adults and to
mortality increase. Nevertheless, these results have not been extrapolated to a
relation with Fragility so far, what indicates further studies should be done
[17].
According to Longo et al. [1], less than 50% of patients with PAD
are symptomatic. However, symptomatology must be considered in the relation
found. The intermittent claudication associated with cramps, tiredness, pain in
rest or during effort are linked to diminish in walking speed and time,
deterioration in the musculoskeletal function, low quality of life and
intolerance to the exercise [18]. Thus, they may explain the association
between Fragility and PAD.
The other clinical and sociodemographic variables assessed and the
associations with Fragility found also regard special attention.
It is verified that 64.7% of frail and 72.2% of pre-frail are women,
expressing a significative difference when compared to men in similar clinical
framework. According to Fried et al. [4], such difference is due to the lower
thin mass percentage and to muscle strenght in female
gender, besides the higher tendency to sarcopenia, anorexia and food
inadequacies among women.
In our sample, 39.5% of the individuals were functional illiterate,
which is more than the national average of 27% [18]. According to Ribeiro et
al. [20], low level of education is prevalent among users of Unique Health
System (UHS in English and SUS in Portuguese), which may explain the difference
from the national average, which considers individuals cared by public and
private service. Similarly, the author observes that UHS low income users
present a worse self-health report. It is also verified that a low level of
education might be a difficult factor to know diseases and to join to suitable
treatments, which makes health worse [21]. Besides, the association between
frailty and low level of education was also found by Duarte et al. [9]
who found a 15.4% higher proportion of frailty among illiterate elderly, and by
Wanaratna et al. [22] who checked Fragility
was 4.04% more frequent in elderly, low level of education women.
Besides the smaller opportunities to health care access, this higher
prevalence might be explained by low nutritional intake [22]. Arterial
hypertension, diabetes and chronic kidney disease also expressed high prevalences. However, the predominance was already expected
because it is a reference center, which cares the population with these
pathologies. Despite of this fact, only the systemic arterial hypertension was
considered significative in frailty incidence. The arterial hypertension was
identified by Benetos et al. [27], in
literature review, as presenting high rates of morbidity and mortality among
frail individuals.
Frailty is related to diminish of physiological reservations, causing
difficulties to maintain homeostasis [4]; this way, Buto
et al. [23] analyzed baroreflex, one of the cardiovascular homeostasis
mechanisms in frail people. The baroreflex presented decoupling between the
cardiac period and systolic arterial pressure in frail and pre-frail people
compared to non-frail ones [23]. There are few studies that verified the
relation between arterial hypertension and frailty. Among them, Newman et
al. [25] checked there is a 15% increase of fragility risk for each 10mmHG
increase of systolic arterial pressure. For Fattori
[26], the arterial hypertension potentially explains alteration of blood flow
to the tissues, accelerating the sarcopenia proccess,
which is a decisive variable of frailty framework. Benetos
et al. [27] reported in a longitudinal study that the use of
anti-hypertension therapy may bring risks to frail elderly, increasing the mortality
rate.
Dyslipidemias are considered any alteration of lipoprotein reference
values, considering total cholesterol, triglycerides, low- and high-density
lipoprotein (LDL-c and HDL-c) and non-HDL cholesterol [28]. The association
found in our study does not point relation between frailty and dyslipidemia.
According to Bastos-Barbosa et al. [24], there are few studies which verified
the relations between these variables, and these ones have not presented
positive associations so far. The exception seems to be with HDL-c. For
example, the study named “iLSIRENTE study” evaluated
359 individuals and showed worse functional performance and mortality by all
causes associated to lower HDL levels in frail elderly [29].
This information was confirmed by Chanti-Ketterl
et al. [30] who reported lower functional capacity in elderly women with
lower HDL levels. Although the important association among PAD, clinical and
sociodemographic variables with Fragility, our work is limited. The
insufficient sample of altered ABI patients made the group division impossible
with values inferior than 0.9 and superior than 1.3. However, the same share
strong association with inflammation, oxidative stress, endotelial
function deterioration, besides the heart, cerebrovascular disease prognosis
and high level of mortality. Another limiting factor is related to the
cross-sectional character of the study, which prevents us from determining
casual relation among the study variables. The data collection through medical
report make a better explanation of the studied associations impossible.
Conclusion
We checked that, among the individuals with ABI alterations, there is a
high prevalence of frailty. The inflammatory state, the oxidative stress and
deterioration of endothelial function are found as main factors that resulted
the association between the two syndromes. Efforts headed to an early detection
of fragility are needed as well as interventions, aiming an improvement of both
frameworks. The clinical and sociodemographic factors which have association
were hypertension and dyslipidemias, gender and level of education,
respectively, and they may indicate the necessity of primary intervention.
Thus, the association between frailty and autonomy significative negative
outcomes, life quality and mortality reinforce the necessity of a continuous
search for comprehension of their pathophysiological basis.
Acknowledgments
The authors thank to Nephrology Treatment, Research, Study
Interdisciplinary Center of Federal University of Juiz de Fora, state of Minas
Gerais, Brazil (NIEPEN) for providing space and information to conduct the
present work.
Funding
This research had no funding.
References
- Longo DL, Kasper DL,
Jameson JL, Fauci AS, Hauser
SL, Loscalzo J. Medicina Interna de Harrison. Porto Alegre: AMGH; 2013.
- Golomb BA, Dang TT, Criqui
MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006;114(7):688-99.
https://doi.org/10.1161/CIRCULATIONAHA.105.593442
- Coelho TC. Risco
cardiovascular, adesão ao tratamento medicamentoso anti-hipertensivo e
Fragilidade em idosos hipertensos [Dissertação]. Campinas: Universidade
Estadual de Campinas. Faculdade de Ciências Médicas; 2013.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch
C, Gottdiener J et al. Frailty
in older adults: evidence for a phenotype. J Gerontol
A Biol Sci Med Sci 2001;56(3):M146-M157.
https://doi.org/ 0.1093/gerona/56.3.m146
- Chen
X, Mao G, Leng SX. Frailty syndrome: an overview.
Clin Interv Aging 2014;9:433.
https://doi.org/10.2147/CIA.S45300
- Afilalo J. Frailty in patients
with cardiovascular disease: why, when, and how to measure. Current Cardiovasc
Risk Reports 2011;5(5):467.
- Makdisse M, Pereira
AC, Brasil DP, Borges JL, Coelho GLM, Krieger JE. Prevalence
and risk factors associated with peripheral arterial disease in the Hearts of
Brazil Project. Arqui Bras Cardiol 2008;91(6):402-14.
https://doi.org/10.1590/S0066-782X2008001800008
- Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl
J Med 2001;344(21):608-1621.
https://doi.org/10.1056/NEJM200105243442108
- Duarte Y, Nunes DP,
Andrade FB, Corona LP, Brito TRP, Santos JLF, Lebrão ML. Fragilidade em idosos
no município de São Paulo: prevalência e fatores associados. Rev Bras Epidemiol 2019;21. https://doi.org/10.1590/1980-549720180021.supl.2
- Manfredi
G, Midão L, Paúl C, Cena C,
Duarte M, Costa E. Prevalence of frailty status among the European elderly
population: Findings from the Survey of Health, Aging and Retirement in Europe.
Geriatr Gerontol Int
2019;19(8):723-9. https://doi.org/10.1111/ggi.13689
- Lang
P, Michel J, Zekry D. Frailty syndrome: A
transitional state in a dynamic process. Gerontology 2009;55(5):39-49.
https://doi.org/10.1159/000211949
- Trevisan C, Veronese N,
Maggi S, Baggio G, Toffanello ED, Zambon
S et al. Factors influencing transitions between frailty states
in elderly adults: The Progetto Veneto Anziani Longitudinal Study. J Am Geriatr
Soc 2017;65(1):179-184. https://doi.org/10.1111/jgs.14515
- Xue Q, Qin MZ, Jia J, Liu JP, Wang Y.
Association between frailty and the cardio-ankle vascular index. Clinical
Intervention in Aging 2019;14:735.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6487894/
- Schaap
LA, Pluijm SMF et al. Higher inflammatory
marker levels in older persons: associations with 5-year change in muscle mass
and muscle strength. J Gerontol A Biol Sci Med Sci 2009;64(11):1183-9.
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch
CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of
frailty: the Cardiovascular Health Study. Arch Intern
Med 2007;167(7):635-41. https://doi.org/
10.1001/archinte.167.7.635
- Alvim RO. Impacto
de marcadores genéticos no fenótipo de rigidez arterial em uma população geral
[Tese]. São Paulo: Universidade de
São Paulo; 2012.
- Zaslavsky O, Cochrane BB,
Thompson HJ, Woods NF, Herting JR, LaCroix A.
Frailty: a review of the first decade of research. Biol Res Nurs 2013;15(4):422-32.
https://doi.org/10.1177/1099800412462866
- Silva
DK, Nahas MV. Prescrição de exercícios físicos para pessoas
com doença vascular periférica. Rev Bras Ciênc Mov
2008;10(1):55-61.
- Lima A, Ribeiro VM, Catelli JR, Roberto. Indicador de Alfabetismo
Funcional–INAF: Estudo especial sobre alfabetismo e mundo do trabalho. São
Paulo: Instituto Paulo Montenegro: Ação Educativa, 2016. [citado 2019 Jun 12]. Disponível em:
http://acaoeducativa.org.br/wpcontent/uploads/2016/09/INAFEstudosEspeciais_2016_Letramento_e_Mundo_do_Trabalho.pdf
- Silva ZPD, Ribeiro
MCSDA, Barata RB, Almeida MFD. Perfil sociodemográfico e padrão de utilização
dos serviços de saúde do Sistema Único de Saúde (SUS), 2003-2008. Ciênc Saúde Colet 2011;16:3807-16.
- Myers
V, Drory Y, Goldbourt U,
Gerber Y. Multilevel socioeconomic status and incidence of frailty post
myocardial infarction. Int J Cardiol
2014;170(3):338-43. https://doi.org/10.1016/j.ijcard.2013.11.009
- Wanaratna K, Muangpaisan
W, Kuptniratsaikul V, Chalermsri
C, Nuttamonwarakul A. Prevalence and Factors
Associated with Frailty and Cognitive Frailty Among Community-Dwelling Elderly
with Knee Osteoarthritis. J Community Health 2019;44(3):587-95.
https://doi.org/10.1007/s10900-018-00614-5
- Buto MSS, Catai
AM, Vassimon-Barroso V, Gois
MO, Porta A, Takahashi AC. Baroreflex sensitivity in frailty syndrome. Braz J Med Biol Res 2019;52(4).
https://doi.org/10.1590/1414-431x20198079
- Bastos-Barbosa RG, Ferriolli E, Coelho EB, Moriguti
JC, Nobre F, Costa Lima NK. Association of frailty
syndrome in the elderly with higher blood pressure and other cardiovascular
risk factors. Am J Hypertens 2012;25(11):1156-61.
https://doi.org/10.1038/ajh.2012.99
- Newman
AB, Gottdiener JS, Mcburnie
MA, Hirsch CH, Kop WJ, Tracy R et al. Associations of subclinical
cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001;56(3):M158-M166.
https://doi.org/10.1093/gerona/56.3.m158
- Fattori A, Santimaria
MR, Alves RMA, Guariento ME, Neri AL. Influence of blood pressure profile on frailty phenotype in
community-dwelling elders in Brazil–FIBRA study. Arch Gerontol
Geriatr 2013;56(2):343-9.
https://doi.org/10.1016/j.archger.2012.08.004
- Benetos, A et al. Treatment
with multiple blood pressure medications, achieved blood pressure, and
mortality in older nursing home residents: the PARTAGE study. JAMA Intern Med 2015;175(6):989-95.
https://doi.org/10.1001/jamainternmed.2014.8012
- Xavier HT, Izar MC, Faria
Neto JR, Assad MH, Rocha VZ, Sposito AC et al. V
Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq Bras Cardiol
2013;101(4):1-20.
- Landi F, Russo A, Cesari
M, Pahor M, Bernabei R, Onder G. HDL-cholesterol and physical performance: results
from the ageing and longevity study in the sirente
geographic area (ilSIRENTE Study). Age Ageing
2007;36(5):514-20. https://doi.org/ 10.1093/ageing/afm105
- Chanti-Ketterl M, Gamaldo
A, Andel R, Thorpe Junior RJ. The association between
lipoproteins, disability, and physical function among older Costa Rican adults.
J Aging Health 2018;30(5):758-77.
https://doi.org/10.1177/0898264317690866