REVIEW
Pathophysiology
of worsening lung function in COVID-19
Fisiopatologia da piora
da função pulmonar no COVID-19
Giulliano Gardenghi,
PhD1,2,3,4
1Scientific Coordinator
of Hospital ENCORE, Aparecida de Goiânia/GO
2Scientific Coordinator
of the CEAFI Faculty, Goiânia/GO
3Technical Consultant of
Lifecare / HUGOL – Burns Intensive Care Unit, Goiânia/GO
4Technical Consultant at São Cristóvão
Hospital and Maternity, São
Paulo/SP
Corresponding author: Giulliano
Gardenghi, Hospital ENCORE, Rua Gurupi, Quadra 25,
Lote 6 a 8 Vila Brasília 74905-350 Aparecida de Goiânia GO Brazil
E-mail:
ggardenghi@encore.com.br
Abstract
Introduction: The new coronavirus pneumonia (COVID-19) has emerged as the main
threats to global health since December 2019. Addressing part of the pulmonary
pathophysiology involved in the disease is important to help interested health
professionals better understand the different aspects of this complex
pathology. Aim: This article aims to present part of the
pathophysiological process involved in pulmonary complications associated with
Covid-19. Methods: An integrative literature review was carried out,
with articles published between 2019 and 2020, in the Google and PubMed
databases, using the following search terms: coronavirus, COVID-19, pulmonary
complications, pneumonia. Results: 6 articles were included, addressing
the proposed theme. Conclusion: The individual's infection with COVID-19
has the potential to cause significant changes in ventilatory capacity, leading
to diffuse pulmonary impairment and worsening gas exchange. Further studies are
needed to clarify the pathophysiology of this complex disease with a high
potential for contagion, morbidity and mortality.
Keywords: coronavirus
infections, communicable diseases, pneumonia.
Resumo
Introdução: A nova pneumonia por coronavírus (COVID-19) surgiu como as principais ameaças à
saúde global desde dezembro de 2019. Abordar parte da fisiopatologia pulmonar
envolvida na doença é importante para ajudar os profissionais de saúde
interessados a compreender melhor os diversos aspectos dessa complexa
patologia. Objetivo: Esse artigo tem o intuito de apresentar parta do processo
fisiopatológico envolvido nas complicações pulmonares associadas à Covid-19. Métodos:
Foi realizada uma revisão integrativa da literatura, com artigos publicados
entre 2019 e 2020, nas bases de dados Google e PubMed,
utilizando os seguintes termos para pesquisa: coronavirus,
COVID-19, complicações pulmonares, pneumonia. Resultados: Foram
incluídos 6 artigos, abordando o tema proposto. Conclusão: A infecção do
indivíduo pela Covid-19 tem potencial de causar alterações significativas na
capacidade ventilatória, cursando com comprometimento pulmonar difuso e piora
nas trocas gasosas. Mais estudos são necessários para esclarecer a
fisiopatologia dessa doença complexa com alto potencial de contágio, morbidade
e mortalidade.
Palavras-chave: infecções por coronavirus, doenças transmissíveis, pneumonia.
Introduction
COVID-19, or the coronavirus, started in
China in late 2019 as a set of cases of pneumonia with an unknown cause. The
cause of pneumonia was found to be a new virus - severe acute respiratory
syndrome coronavirus 2 or Sars-CoV-2. Now declared a pandemic by the World
Health Organization (WHO), most people who contract COVID-19 suffer only mild
symptoms. The WHO says that only one person in six becomes seriously ill
"and will develop difficulty in breathing". Almost all
of the serious consequences of COVID-19 have pneumonia. The WHO also
says that elderly people and people with underlying problems, such as high
blood pressure, heart and lung problems or diabetes, are more likely to develop
serious illnesses [1].
Regularly, when people with COVID-19
develop cough and fever, this is a result of the infection that affects the
bronchial tree. The lining of the bronchi is injured, causing inflammation.
This, in turn, irritates the nerves in the lining of the airways, and in such
situations, just a grain of dust can stimulate coughing. With the evolution of
the condition, the virus reaches the gas exchange units (alveoli), igniting
them and, consequently, promoting the filling of such alveoli by liquids,
cellular debris and others, due to the alterations caused in the
alveolar-capillary membrane. This condition will therefore be characterized as
pneumonia, resulting in an inability of gas exchange with consequent hypoxemia
and hypercapnia. Pneumonic conditions are associated with mortality, especially
in the elderly [1].
Chen et al. [2] retrospectively
studied 99 patients with pneumonia caused by COVID-19. The average age of the
patients was 55.5 years, including 67 men and 32 women. 51% of patients had
chronic diseases. The patients presented clinical manifestations of fever
(83%), cough (82%), shortness of breath (31%), muscle pain (11%), mental
confusion (9%), headache (8%), headache throat (5%), rhinorrhea (4%), chest
pain (2%), diarrhea (2%) and nausea and vomiting (1%). According to the imaging
exam, 75% of patients had bilateral pneumonia, 14% of patients had multiple
spots and ground-glass opacity, and 1% of patients had pneumothorax. 17% of
patients developed acute respiratory distress syndrome (ARDS) and, among them,
11% of patients worsened in a short period and died from multiple organ
failure.
Methods
An integrative literature review was
carried out, with articles published between 2019 and 2020, in the Google and
PubMed databases, using the following search terms: coronavirus, COVID-19,
pulmonary complications, pneumonia, and 06 articles were selected for writing
the present manuscript.
Histopathological
findings
Luo et al. [3] describe, in data
not yet published, histopathological findings related to a 66-year-old male
patient who had symptoms of high fever and cough when he returned to Shenzhen
City, coming from Wuhan on January 4, 2020 This individual had only
hypertension as a comorbidity. On macroscopic examination (Figure 1), the
surface of the entire lung showed a diffuse congestive appearance. There was
punctual hemorrhage and partially hemorrhagic necrosis. Hemorrhagic necrosis
was present mainly on the outer edge of the lower right lobe, middle lobe and
upper lung lobe. The bronchi were swollen and the
mucous surfaces were covered with hemorrhagic exudation. The cut surfaces of
the lung showed severe congestive and hemorrhagic changes.
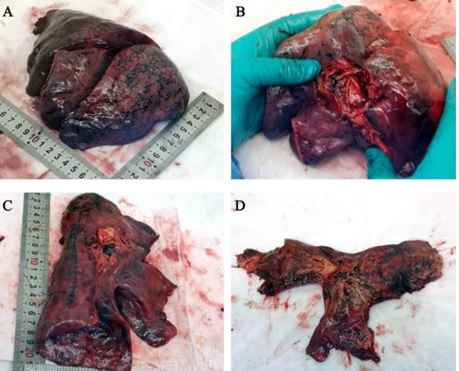
Figure
1 - Macroscopic lung examination at COVID-19.
Morphology of the right lung (Panel A and B) and the left lung (Panel C and D).
Hemorrhagic necrosis is obvious at the outer edge of the right pulmonary lobe.
Image reproduced from the data of Luo et al. [3].
As shown in Figure 2, histopathological
findings showed extensive interstitial fibrosis with partially hyaline
degeneration and pulmonary hemorrhagic infarction. The small vessels showed
hyperplasia, thickening of the vessel wall and stenosis / occlusion.
Interstitial infiltration of inflammatory cells, including lymphocytes and
mononuclear cells. Pulmonary interstitial fibrosis was confirmed, and no other
bacterial and fungal infections were found by special staining.
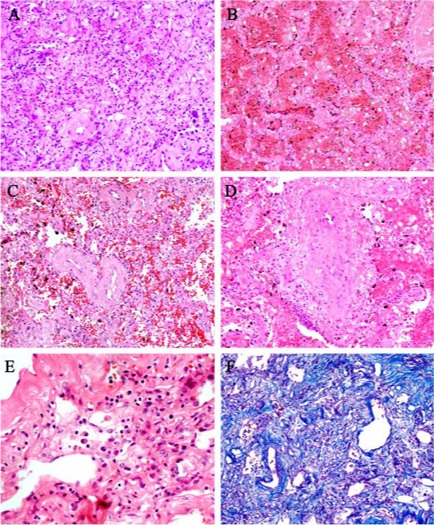
Figure
2 - Pulmonary interstitial histopathology associated
with a critical patient in COVID-19. A: Massive pulmonary interstitial
fibrosis. B: Pulmonary hemorrhagic infarction. C: Vascular wall thickening and
stenosis of the lumen. D: Thromboangiitis Obliterans
surrounded by inflammatory cells. E: Infiltrated interstitial plasma cells. F:
Pulmonary interstitial fibrosis. Image reproduced from the data of Luo et al.
[3].
There was alveolitis with atrophy,
proliferation, desquamation and various changes in the squamous metaplasia of
alveolar epithelial cells (mainly type ?), as listed
in Figure 3. The remaining pulmonary alveoli showed a thickened septum,
necrosis and desquamation of alveolar epithelial cells. In addition, massive
fibrous exudate, giant multinucleated cells and intracytoplasmic viral
inclusion bodies. Necrotizing bronchiolitis and manifest necrosis of the
bronchiolar wall, with epithelial cells present in the lumen.

Figure
3 - Changes in the pulmonary alveoli of COVID-19. A:
Necrotizing bronchiolitis, necrotic bronchial epithelial cells are present in
the lumen. B: Atrophy of alveolar epithelial cells. C and D: Various changes in
squamous metaplasia of alveolar cells. E: Thickened alveolar septum. F:
Necrosis and desquamation of alveolar epithelial cells. G: massive fibrinous
exudate in the lumen. H: Multinucleated giant cell. I: Intracytoplasmic viral
inclusion body in an alveolar epithelial cell (indicated in the box). Image
reproduced from the data of Luo et al. [3].
Chart
I - Summary of anatomical and pulmonary
histopathological findings in COVID-19, based on the study by Luo et al. [3].
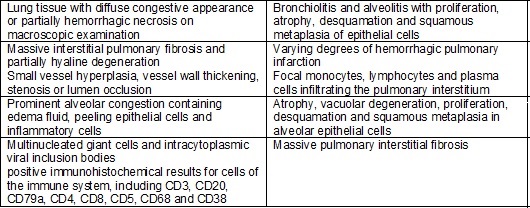
Abnormalities
seen on chest tomography (CT)
COVID-19 pneumonia manifests with
abnormalities in chest CT, even in asymptomatic patients, with rapid evolution
of unilateral to diffuse bilateral ground-glass opacities that evolve or
coexist with consolidations in 1-3 weeks. Combining the assessment of imaging
resources with clinical and laboratory findings can facilitate the early
diagnosis of COVID-19 pneumonia [4].
Abnormalities
in chest CT before symptoms
Shi et al. [4] retrospectively
reviewed the chest CT findings of 81 patients with confirmed COVID-19. Patients
were subdivided into 4 groups based on the duration of clinical symptoms. Group
1 consisted of 15 patients who underwent a chest CT scan before any clinical
symptoms; group 2 underwent a CT scan within 7 days after the onset of
symptoms; group 3 patients were examined 7 to 14 days after the onset of
symptoms. It is important to note that all 81 patients [including those without
symptoms] had an abnormal chest CT consistent with viral pneumonia. In the
asymptomatic group, the typical pattern was ground-glass, multifocal and
peripheral opacities (figure 4). Thickening of the interlobular septum,
thickening of the adjacent pleura, nodules, round cystic changes,
bronchiectasis, pleural effusion and lymphadenopathy were rarely observed in
the asymptomatic group.

Figure
4 - Illustration of the evolution of chest CT during
COVID-19. Hypothetical initial stage with bilateral, multifocal and predominantly
peripheral ground-glass opacity. Source: author's archive image.
Still analyzing the study by Shi et al.
[4], there was radiographic progression after the first symptoms. In group 2
(that is, in the first 7 days of symptoms), CT chest lesions became bilateral
in 90% and diffuse in more than 50%, predominantly with ground-glass opacities
(figure 05). Pleural effusion and some cases of lymphadenopathy were also
detected in group 2. In group 3 (ie, 7 to 14 days
after symptoms), the ground-glass aspect was still the predominant finding on
CT in more than 50% of cases, however, consolidation patterns were also seen in
about a third of patients. Finally, in group 4 (that is, more than 14 days
after symptoms), ground-glass opacities and reticular patterns were more
common.
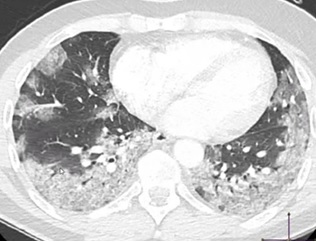
Figure
5 - Illustration of the further evolution of
ground-based opacities in ground glass in COVID-19. The lesions are now
bilateral and multifocal. Source: author's archive image.
Association
of COVID-19 with hemoglobin
Wenzhong & Hualan [5] released the results of their study, mentioning
that in the viral replication phase after entering a person's organism,
coronavirus RNA encodes the production of structural proteins (for the
structure of the virus) and other non-structural proteins. One of these
non-structural proteins invades hemoglobins, removes
the iron atom and binds at the site, preventing oxygen from being carried. This
would explain the rapidly evolving hypoxia picture. They postulate that the
lung parenchyma lesions (ground glass) are a consequence of hypoxia and
consequent necrosis and not a direct effect of the inflammatory process caused
by the virus. This could explain people with comorbidities, especially
diabetes, who decompensate quickly due to hypoxia, sometimes even with
supplemental oxygen supply, as these individuals would have fewer binding sites
in hemoglobins. Theoretically, in people without
comorbidities, the initial viral load would be responsible for determining the
severity of the condition since the higher the viral load, the more
theoretically there are compromised hemoglobins. It
is also suggested that the change in the structure of red blood cells would
explain vessel damage and disseminated intravascular coagulation.
Conclusion
Infection of the individual by
COVID-19 has the potential to cause significant changes in ventilatory
capacity, leading to diffuse pulmonary impairment and worsening gas exchange.
Further studies are needed to clarify the pathophysiology of this complex
disease with high potential for spread, morbidity and mortality.
References
- World
Health Organization - WHO. Coronavirus disease (COVID-19) Pandemic [accessed in
Apr 10 2020]; Available in:
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Chen
N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang
J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan,
China: a descriptive study. Lancet. 2020;395(10223):507-13.
https://doi.org/10.1016/S0140-6736(20)30211-7
- Luo
W, Yu H, Gou J, Li X, Sun Y, Li J, Liu L. Clinical Pathology of Critical
Patient with Novel Coronavirus Pneumonia (COVID-19). Preprints 2020, 2020020407
[periódicos na Internet]. [acesso em 10 abr 2020];
Disponível em: https://www.preprints.org/manuscript/202002.0407/v1
- Shi
H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y,
Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in
Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20(4):425-34.
https://doi.org/10.1016/S1473-3099(20)30086-4
- Wenzhong L, Hualan
L. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin
to Inhibit Human Heme Metabolism. ChemRxiv 2020; Preprint. https://doi.org/10.26434/chemrxiv.11938173.v6