Rev Bras Fisiol Exerc 2021;20(3):378-87
doi: 10.33233/rbfex.v20i3.4205
ARTIGO ORIGINAL
Effect of α-lipoic acid supplementation on
oxidative stress of heart and liver tissues of endurance-trained mice submitted
to an exhaustive exercise
Efeito
da suplementação de ácido α-lipóico no estresse
oxidativo de tecidos cardíaco e hepático de camundongos treinados em endurance submetidos a um exercício exaustivo
Letícia
Santana Wolf1, Álisson de Carvalho
Gonçalves2, Ruan Carlos Macedo de Moraes3, Ana Carolina
Nunes Rodrigues4, Susana Merino4, Guilherme Vannucchi Portari5
1Departamento de Ciências da Saúde,
Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão
Preto, SP, Brasil
2Instituto Federal de
Educação, Ciência e Tecnologia Goiano, Campus Urutaí,
Urutaí-GO, Brasil
3Departamento de Fisiologia, Instituto de
Ciências Biomédicas, Universidade de São Paulo, São Paulo, SP, Brasil
4Laboratório de Nutrição Experimental,
Instituto de Ciências da Saúde, Universidade Federal do Triângulo Mineiro,
Uberaba, MG, Brasil
5Departamento de Clínica Médica,
Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão
Preto, SP, Brasil
6Departamento de Nutrição, Instituto de
Ciências da Saúde, Universidade Federal do Triângulo Mineiro, Uberaba, MG,
Brasil
Received: June 15, 2020; Accepted: May 4, 2021.
Correspondence: Guilherme Vannucchi Portari, Av. Getúlio Guaritá,
159/121 Abadia 38025-440 Uberaba MG, Brasil
Letícia Santana Wolf: leticiaswolf@gmail.com
Álisson de Carvalho Gonçalves: alisoncg88@hotmail.com
Ruan Carlos Macedo de Moraes: moraes.ruan@gmail.com
Ana Carolina Nunes Rodrigues: anacarol-vet@hotmail.com
Susana Merino: smerino.nutri@hotmail.com
Guilherme Vannucchi Portari: guilherme.portari@uftm.edu.br
Abstract
Introduction: High intensity
exercise causes an increase in reactive oxygen species production, which can be
harmful to the health and function of several organ tissues. Objective:
To analyze the effect of supplementation with α-lipoic acid against the
oxidative stress in the heart and liver of endurance-trained mice submitted to
the exhaustive endurance exercise bout. Methods: Thirty-two male mice
were submitted to 6-week endurance swimming training, and divided in two groups
according to supplementation protocol, α-lipoic acid
or vehicle, during the last two weeks. The last training session was destined
to the exhaustive exercise bout. It was analyzed the lipid peroxidation,
oxidative damage to proteins and antioxidant marker in liver and heart
immediately (0h) and four-hours (4h) after the exhaustive exercise in both
groups. Results: The heart of supplemented animals showed a lower
protein damage and higher levels of antioxidant in 0h and 4h. In the liver,
lipid peroxidation was higher in supplemented animals in 0h but did not differ
4h after the exhaustion. The liver of supplemented animals showed higher levels
of carbonylated protein in both 0h and 4h. Conclusion: The α-lipoic
acid supplementation is an efficient antioxidant to the heart of trained mice
submitted to exhaustive exercise but is unnecessary to avoid exhaustion-induced
oxidative stress in the liver.
Keywords: physical exercise; oxidative
stress, lipoic acid
Resumo
Introdução: O exercício de alta intensidade
promove um aumento na produção de espécies reativas, o que pode ser prejudicial
para a saúde e função de diversos órgãos. Objetivo: Analisar o efeito da
suplementação com ácido α-lipóico contra o estresse oxidativo no coração e no fígado
de camundongos treinados em endurance submetidos a
uma sessão de exercício exaustivo. Métodos: Trinta e dois camundongos
machos foram submetidos a 6 semanas de treinamento em natação. Os animais foram
divididos em dois grupos de acordo com a suplementação, ácido α-lipóico ou
veículo, oferecida durante as duas últimas semanas. A última sessão do
treinamento foi destinada ao exercício de exaustão. Foram analisadas a
peroxidação lipídica, dano oxidativo às proteínas e marcador antioxidante no
fígado e coração imediatamente (0h) e quatro horas (4h) após o exercício
exaustivo nos dois grupos. Resultados: O coração dos animais
suplementados apresentou menor dano proteico e maiores níveis de antioxidante
nas 0h e 4h. No fígado, a peroxidação lipídica foi maior nos animais
suplementados em 0h, mas não diferiu 4h após a exaustão. O fígado dos animais
suplementados apresentou níveis mais altos de proteína carbonilada
nas 0h e nas 4h. Conclusão: A suplementação com ácido α-lipóico é um
antioxidante eficiente para o coração de camundongos treinados submetidos a
exercício exaustivo, mas é desnecessário para evitar o estresse oxidativo
hepático induzido pelo esforço exaustivo.
Palavras-chave:
exercício físico; estresse oxidativo; antioxidante
The aerobic
metabolism is the main reactive oxygen species (ROS) endogenous source. The
oxidative phosphorylation produces many superoxide anions, which can form other
reactive species, like as hydrogen peroxide and hydroxyl radical [1]. However,
in physiological conditions, the ROS are neutralized by a complex endogenous
antioxidant system, which is composed of enzymatic and non-enzymatic
antioxidants. The antioxidant molecules are extremely important to avoid
oxidative damage to cell components, such as proteins, nucleic acids, and
lipids [2,3].
High intensity
or strenuous physical exercise bouts cause an expressive increase in ROS
production [1]. Besides exercise is a potential stimulus to trigger ROS
production, the scientific literature has shown that an adequate physical
exercise-training program has a protective effect against oxidative damage,
since it enhanced the antioxidant system [4]. However, it has been shown that
high production of ROS and impairment in the antioxidant system can limit the
exercise adaptation and performance [1,5]. Chronic exposition to high ROS
levels can significantly decrease the activity of enzymatic antioxidant system
(superoxide dismutase, catalase, glutathione peroxidase, etc.) and the
concentration of non-enzymatic antioxidant (coenzyme Q10, glutathione, vitamin
C and E, lipoic acid, etc.), impairing the cellular function by triggering
damage, apoptosis, and necrosis [2].
The oxidative
damage from exercise in trained muscle has been largely studied since the
muscle is the most recruited organ during an exercise bout. However, other
organs have an increase in their activity during and immediately after an
exercise bout, especially heart and liver [6,7]. Studies have shown negative
alterations in the redox status of the heart and liver after strenuous exercise
[8,9]. Fortunately, it has been studied nutritional strategies able to prevent
and/or reduces oxidative damage, and consequently, reduces physical stress,
muscle pain, and impairments to sports performance [10]. Some studies have
shown that the intake of exogenous antioxidant molecules has positive effects
against exercise-induced oxidative damage [8,11].
The α-lipoic
acid is cofactor to mitochondrial enzymes involved in energy metabolism also,
play a role in ROS neutralization and metal chelation [12]. The α-lipoic
acid has been considered universal antioxidant since it acts both in the
aqueous phase and in the membrane, act in synergy with other antioxidants (such
as glutathione, vitamin C and vitamin E). Also, it can recycle other
antioxidants such as ascorbic acid, glutathione (GSH) and vitamin E [12,13].
Studies have shown that α-lipoic acid supplementation can improve
antioxidant defense and reduces oxidative damage in muscle tissue after an
exercise bout [14,15].
Thus, the
present study aimed to analyze the effect of supplementation with α-lipoic
acid against oxidative stress in the heart and liver of endurance-trained mice
submitted to the exhaustive endurance exercise bout.
Methods
Animals
Thirty-two
6-week-old male Swiss mice (Mus musculus) were placed in two experimental
groups according to the intervention: 1) Vehicle group (VEH) (n = 16): animals
submitted to exercise training and which did not
receive supplementation; 2) Supplemented group (SUP) (n=16): animals submitted
to exercise training and which received supplementation. The animals were
housed in plastic cages, in an inverted circadian cycle (12-h dark/light), in
22 ± 1 °C and 55 ± 5% humidity with feeding and tap water ad libitum, in the
animal facility of the Laboratory of Experimental Nutrition of Federal
University of Triangulo Mineiro
(UFTM). The experiment protocol had the previous authorization of the Ethics
Committee of Animal Use of UFTM, under protocol number 219/12.
Supplementation protocol
The α-lipoic
acid (Zhejiang Chemicals, China) was diluted (1 mg/mL) in a vehicle solution
(10% dimethyl sulfoxide (DMSO) in soybean oil). The solution was administered
by gavage (100 mg/kg/day) during the last seven days of the exercise training
protocol, in the animals of the SUP. The VEH received the same volume of the
vehicle solution during the last seven days of the exercise training protocol.
Exercise training protocol
The exercise
training protocol was applied according to proposed by Sampaio-Barros et al.
[16]. The first week was destined for acclimation to the exercise training
protocol. The animals were submitted to swimming training for 5-minutes on the
first day, 15-min in the second, 30-min in the third day, 45-min, and 60-min in
the fourth and fifth days, respectively. Then, the animals were daily submitted
to a swimming session, 5 days per week, 60-min per session, during 6-weeks. The
training session was applied in groups of 5 animals aimed to increase the
intensity of exercise [16]. Animals swam in a plastic container of 22 cm
diameter and 60 cm height, with a tap water depth of 40 cm, maintained at 32°C
(± 1°C) controlled by the heater with an automatic thermostat (HOPAR SA-333
Zhong Shan, China).
The last session
was dedicated to the exhaustive exercise bout. The animals were submitted to an
individual swimming session with a metal load corresponding to 10% of
bodyweight attached to the proximal portion of the tail. Animals were exhausted
when they were unable to support the snout in the surface of the water for 8
seconds [17].
Sample collection and preparation
The animals were
euthanized by decapitation after being anesthetized with ketamine (80 mg/kg)
and (5 mg/kg) xylazine immediately after (0h) the exhaustive exercise (n = 8)
and four-hours after (4h) the exhaustive exercise (n = 8). After the euthanasia
confirmation, the liver and heart were immediately excised, washed in saline
solution, and immediately frosted in liquid nitrogen. The organs were kept at
-20 °C until the time of analyzes. Aliquots of heart and liver tissue were
homogenized with 25 mM phosphate buffer pH 7.4 (1:100 w/vol) immediately before
the analyses start.
Determination of carbonylated proteins
The
concentration of carbonylated proteins was determined by the method proposed by
Odetti et al. [18]. Homogenate tissue (100 µL)
was vigorously mixed with 100 µL 20% tricarboxylic acid (TCA) and centrifugated
for 10 minutes at 3500 rpm. The supernatant was discarded and 500 µL of 10 mM
2,4-dinitrophenylhydrazine (DNPH), diluted in 2M HCl, was added to precipitate.
The solution was incubated for 1 hour at room temperature in the dark, with
shaking every 15 min. Then, it was added 1 mL of ethanol-ethyl acetate (1:1
vol/vol) was added and the solution was centrifugated for 10 min at 3500 rpm at
4ºC. Ethanol-ethyl acetate was removed, and the pellet was suspended in 2 mL of
6M guanidine. The protein-guanidine solution was kept in a water bath at 34ºC
for 15 min. The final solution was read in spectrophotometry set at 370 nm, and
the coefficient molar extinction rate 22,000 M-1cm-1 was
used to calculate the carbonyl content.
Determination of lipid peroxidation
The lipid
peroxidation was measured by the concentration of thiobarbituric
reactive substances (TBARS) according to Buege and Austi [19]. It was added 1mL of TCA-TBA-HCL reagent to 500
µL of tissue homogenate. The solution was kept for 15 minutes in boiling water
(100ºC). After cooling, the solution was centrifuged for 10 minutes at 10000 g.
The absorbance of the supernatant was read at 535 nm. The TBARS concentration
was determined using an equation of the calibration curve obtained by a similar
reaction using commercial malondialdehyde solution.
Determination of non-protein thiols
The non-protein
thiols concentration was measured by colorimetric method, using the reaction of
sulfhydryl group with 5.5’dhytiobis (2-nitrobenzoic acid) (DTNB). Tissue
homogenate was deproteinized by adding 10% TCA. It was added 200 µL of 0.2 M
Tris -0.02M EDTA, 300 µL of DTNB, and 1.6 mL of methanol to 100 µL of tissue
homogenate supernatant. The solution was incubated for 15-min at room
temperature and in dark. Then, the absorbance was read with a spectrophotometer
set at a wavelength of 412 nm. The non-protein thiols concentration was
determined using an equation of the calibration curve obtained by a similar
reaction using commercial GSH solution [20].
Statistical analysis
The data are
presented as mean ± standard deviation. The results were analyzed using the
software SPSS 20.0. To check the variances' equality and data distribution, Levene's test and Shapiro-Wilk test, respectively, were
applied. The data were compared by the analyzes of variance (ANOVA) two-way and
Tukey’s post hoc. A significance level of 95% (p < 0.05) was adopted.
Results
The heart tissue
of the VEH group showed a lower concentration of non-protein thiols in 0h-time
compared to 4h. Similarly, the heart of supplemented animals showed a higher
concentration of non-protein thiols in 4h than in 0h-time. The SUP group showed
a higher concentration of non-protein thiols than the VEH in both experimental
periods (0h, and 4h) (Figure 1A).
The heart tissue
of the SUP group showed higher TBARS concentration compared to the VEH group in
0h-time. However, TBARS concentration was not different between the groups in
4h-time. The TBARS concentration in the heart tissue of both the groups did not
alter through the experimental time (0h to 4h) (Figure 1B).
In 0h and
4-time, the heart tissue of the SUP group showed lower carbonylated protein
concentration than the VEH group. Both the SUP and VEH group did not show any
difference between the experimental periods (Figure 1C).
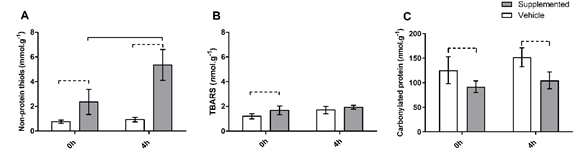
A = Non-protein
thiols; B = Thiobarbituric acid reactive substances
TBARS; C = Carbonylated protein. White bars represent the VEH group. Grey bars
represent the SUP group. Dotted connector indicates an intergroup variation
statistically significant (p < 0.05) at same time (SUP vs. VEH). Whole
connector indicates an intragroup variation statistically significant (p <
0.05) at different times (0h vs. 4h)
Figure 1 - Oxidative
stress markers in heart tissue
In the liver,
the concentration of the non-protein thiols in 0h-time was higher than in
4h-time in both the SUP and VEH groups. There was no difference between groups
in both experimental times (0h and 4h) (Figure 2A).
In supplemented
animals, the TBARS concentration in liver tissue showed a significant reduction
4 hours after the exhaustive exercise. The hepatic TBARS concentration of the
VEH group did not differ through the experimental time. Immediately after
exhaustive exercise bout (0h), the TBARS concentration showed to be higher in
the SUP than in the VEH group. However, there was no difference between the
groups 4-hours after the exhaustive exercise (Figure 1) (Figure 2B).
The
concentration of carbonylated protein of the SUP group was higher than VEH in
both experimental times (0h, and 4h). The liver of the non-supplemented animals
showed higher protein carbonyl content in 4h compared to 0h-time. It was not
finding any difference in carbonylated protein concentration between the times
0h and 4h (Figure 2C).
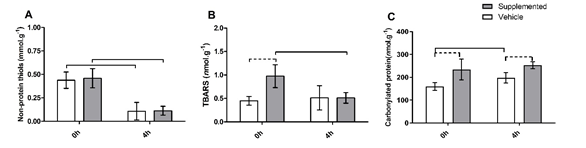
A = Non-protein
thiols; B = Thiobarbituric acid reactive substances
TBARS; C = Carbonylated protein. White bars represent the VEH group. Grey bars
represent the SUP group. Dotted connector indicates an intergroup variation
statistically significant (p < 0.05) at same time (SUP vs. VEH). Whole
connector indicates an intragroup variation statistically significant (p <
0.05) at different times (0h vs. 4h)
Figure 2 - Oxidative
stress markers in liver tissue
Discussion
The present
study aimed to evaluate how the α-lipoic acid supplementation affects the
exhaustive exercise-induced oxidative stress in the heart and liver of
endurance-trained animals after. It was found positive alterations in oxidative
stress markers in the heart tissue of the supplemented animals, especially four
hours after the exhaustive exercise. However, the α-lipoic supplementation
seems to be related to increases in the oxidative damage in the liver.
ROS play an
essential role in cell signaling and function [21]. The increase in the ROS
concentration in the myocardium results in effects on its structure and
functions, such as stimulation of cardiac hypertrophy and apoptosis of
cardiomyocytes, thus contributing to cardiac remodeling [22]. Since the
improvement in heart efficiency is one of the most important exercise
adaptations, the presence of moderate ROS levels seems to be necessary.
However, the excessive ROS attack on macromolecules can be harmful to cell
function and can lead it to apoptosis [23]. Thus, the balance between antioxidant
capacity and pro-oxidant activity must be considered to the maintenance of the
cardiac health and sports performance of individuals engaged in exercise
training programs.
During an
exhaustive effort, the heart workout is highly required. The myocardium energy
demand is mainly supplied by the aerobic metabolism [24]. Thus, the heart
tissue is submitted to intense reactive species attack since the mitochondrial
metabolism is the main source of ROS. However, studies have shown no increases
in oxidative damage in the heart of endurance-trained animals [25,26]. The
present study also did not find any increase in oxidative damage markers in
heart tissue four hours after the exhaustion compared to the results obtained
immediately after the exhaustive effort.
The α-lipoic
acid could have mitigated the harmful effects of exhaustion-induced oxidative
damage in the heart proteins. The heart tissue of supplemented animals showed a
lower concentration of carbonylated protein, which indicates less oxidative
damage to proteins. This could be related to the higher antioxidant
concentration in the supplemented animal. However, similar results could not be
observed in the lipid peroxidation marker of heart tissue. Despite the TBARS
concentration in supplemented animals to be slightly higher than
non-supplemented animals immediately after the effort, the values did not
differ four hours later. It seems that the swimming exhaustive exercise was not
enough stimulus to induce lipid peroxidation in the heart tissue of the trained
mice. In fact, it was shown that exhaustive endurance exercise was able to
alter some oxidative stress markers in the heart tissue of endurance-trained
rats, but not the lipid peroxidation marker [27].
The non-protein
thiols, which are antioxidant molecules, showed in higher levels in the
myocardium of supplemented animals in both the experimental times (0h and 4h).
The α-lipoic acid has a known potential effect to recovery and/or improve
other antioxidant molecules and mechanisms, that can be related to high
antioxidant concentration, especially GSH [12], the main representative of
non-protein thiols [28].
Although
skeletal-muscles and myocardium are widely used during a strenuous and
prolonged effort, the liver work also has a significant increase in this
situation [6]. Some works have shown increases in oxidative damage markers in
liver tissue after exhaustive endurance exercise [6,29]. Several studies have
shown the antioxidants effects of α-lipoic acid in the liver [30,31,32]. An
experimental study found that the administration of α-lipoic acid was
effective in reducing lipid peroxidation and preserving glutathione peroxidase
activity and GSH concentration in the liver of rats subjected to toxic doses
acetaminophen [31]. In non-trained rats, it was showed that α-lipoic acid
supplementation can protect liver cells against oxidative lipid damage promoted
by strenuous exercise bout [30].
In the present
study, it was observed an expressive reduction in antioxidant concentration in
four hours after the exhaustive exercise in both groups. Interestingly, the
antioxidant concentration in 0h and 4h was not different between the groups.
These data indicate that despite the α-lipoic acid play an important role
in antioxidants recycling [12], it is not able to increase the concentration of
non-protein thiols immediately after an exhaustive endurance bout in the liver
tissue of trained mice. In addition, the supplementation protocol failed to
maintain the antioxidant concentration throughout the four hours following the
exhaustion.
It is important
to highlight that the concentration of non-protein thiols suffered a
significant reduction four hours after the exhaustive effort, in both the
experimental groups. This indicates that changes in oxidative stress markers
continue to change for a few hours after the exhaustion bout. Several studies
[33,34,35] have shown that exercise-induced oxidative stress can be observed for a
long time after exercise. In humans, it was shown that the antioxidant capacity
was lower 24 hours after than immediately after a strenuous exercise bout [33].
There was a
modest increase in the oxidative damage to proteins in the liver of the VEH
group. However, the concentration of carbonylated proteins maintained higher in
SUP groups in both the experimental periods. This can be related to the
antioxidant mechanism of α-lipoic acid. Lipoic acid and dihydrolipoic acid (DHLA) (produced from α-lipoic
acid) are reactive to thiol protein compounds [12]. In addition, DHLA can
accelerate iron-dependent hydroxyl radical generation and lipid peroxidation
[13]. Possibly, the same result was not observed in heart because iron
concentration in hepatic tissue is expressively higher than in myocardium [36].
Moreover, the liver cells have a high capacity for uptake and accumulation of α-lipoic
acid metabolites, such as DHLA and lipoate [37].
Immediately
after the exercise, the liver of non-supplemented animals suffered less lipid
peroxidation. The TBARS concentration in the liver showed a modest decrease 4
hours after the effort only in supplemented animals, but the result was not
different than in the non-supplemented animals. Thus, α-lipoic acid seems
to not affect the exhaustive exercise-induced oxidative stress in the liver
tissue of trained mice. The mice’s training level could be related to the
inefficiency of the supplementation protocol proposed in this study. Navarro et
al. [38] showed that moderate exercise training, per si,
decreases the oxidative stress in the liver of middle-aged mice. Other study
[39] found that endurance training promotes liver adaptations, which can
attenuate exhaustive exercise-induced oxidative stress, becoming the
antioxidant supplementation an unnecessary strategy against oxidative stress in
the liver.
Conclusion
The α-lipoic
acid supplementation is effective to increase the antioxidant capacity and to
reduce the oxidative damage in the heart tissue of trained mice after an
exhaustive effort. However, the α-lipoic acid cannot maintain the
antioxidant levels in liver tissue and it was related
to an increase in oxidative damage. Thus, the α-lipoic acid
supplementation is an effective strategy to avoid the exhaustion-induced
oxidative stress in the heart of trained mice but does not in the liver tissue.
References
- Powers SK, Jackson MJ. Exercise-induced oxidative
stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008;88(4):1243-76. doi: 10.1152/physrev.00031.2007 [Crossref]
- Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 2013;51:15-25. doi: 10.1016/j.fct.2012.09.021 [Crossref]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal 2006;8(9-10):1865-79. doi: 10.1089/ars.2006.8.1865 [Crossref]
- Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, et al.
Oxidative stress: role of physical exercise and antioxidant nutraceuticals in
adulthood and aging. Oncotarget 2018;9(24):17181. doi: 10.18632/oncotarget.24729 [Crossref]
- Reid MB. Redox interventions to increase exercise
performance. J Physiol 2016;594(18):5125-33. doi: 10.1113/jp270653 [Crossref]
- Huang C-C, Lin W-T, Hsu F-L, Tsai P-W, Hou C-C.
Metabolomics investigation of exercise-modulated changes in metabolism in rat
liver after exhaustive and endurance exercises. Eur J Appl Physiol
2010;108(3):557-66. doi: 10.1007/s00421-009-1247-7 [Crossref]
- Oláh A, Németh BT, Mátyás C, Horváth EM, Hidi L, Birtalan E, et al. Cardiac effects of acute exhaustive
exercise in a rat model. Int
J Cardiol 2015;182:258-66. doi: 10.1016/j.ijcard.2014.12.045 [Crossref]
- Carvalho
AG, Moreira EJS, Portari GV. Benfotiamine supplementation prevents oxidative stress in anterior
tibialis muscle and heart. J Integr Med 2019;
17(6):423-429. doi: 10.1016/j.joim.2019.07.001 [Crossref]
- Yan F, Wang B, Zhang Y. Polysaccharides from Cordyceps
sinensis mycelium ameliorate exhaustive swimming exercise-induced oxidative
stress. Pharm Biol 2014;52(2):157-61. doi: 10.3109/13880209.2013.820197 [Crossref]
- Pingitore A,
Lima GPP, Mastorci F, Quinones A, Iervasi
G, Vassalle C. Exercise and
oxidative stress: potential effects of antioxidant dietary strategies in
sports. Nutrition 2015;31(7-8):916-22. doi: 10.1016/j.nut.2015.02.005 [Crossref]
- Abadi A, Crane JD, Ogborn D,
Hettinga B, Akhtar M, Stokl
A, et al. Supplementation with α-lipoic acid, CoQ10, and vitamin E
augments running performance and mitochondrial function in female mice. PloS One.
2013;8(4):e60722. doi: 10.1371/journal.pone.0060722 [Crossref]
- Moini
H, Packer L, Saris N-EL. Antioxidant
and prooxidant activities of α-lipoic acid and dihydrolipoic
acid. Toxicol Appl Pharmacol
2002;182(1):84-90. doi: 10.1006/taap.2002.9437 [Crossref]
- Scott BC, Aruoma OI, Evans
PJ, O’neill C, Van Der Vliet A, Cross CE, et al.
Lipoic and dihydrolipoic acids as antioxidants. A
critical evaluation. Free Radic Res
1994;20(2):119-33. doi: 10.3109/10715769409147509 [Crossref]
- Chae
C-H, Shin C-H, Kim H-T. The combination of α-lipoic acid supplementation
and aerobic exercise inhibits lipid peroxidation in rat skeletal muscles. Nutr Res 2008;28(6):399-405. doi: 10.1016/j.nutres.2008.02.010 [Crossref]
- Kinnunen S, Oksala N, Hyyppä S, Sen CK, Radak Z, Laaksonen DE, et al. α-Lipoic acid modulates
thiol antioxidant defences and attenuates
exercise-induced oxidative stress in standardbred trotters. Free Radic Res
2009;43(8):697-705. doi: 10.1080/10715760903037673 [Crossref]
- Sampaio-Barros MM, Farias-Silva E, Grassi-Kassisse DM, Spadari-Bratfisch RC. Effect of swimming session duration and repetition on metabolic markers in rats. Stress 2003;6(2):127-132. doi: 10.1080/1025389031000110169 [Crossref]
- Voltarelli FA, Gobatto CA, De Mello MA: Determination of anaerobic
threshold in rats using the lactate minimum test. Braz J Med Biol
Res 2002;5(11):1389-94. doi: 10.1590/s0100-879x2002001100018 [Crossref]
- Odetti
P, Girabaldi S, Gurreri G, Aragno I, Dapino D, Pronzato MA, et al. Protein
oxidation in hemodialysis and kidney transplantation. Metabolism
1996;45(11):1319-22. doi: 10.1016/s0026-0495(96)90108-0 [Crossref]
- Buege J,
Aust S. Microsomal lipid peroxidation. Methods Enzymol
1978;52:302-10. doi: 10.1016/s0076-6879(78)52032-6 [Crossref]
- Sedlak J,
Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl
groups in tissue with Ellman’s reagent. Anal Biochem 1968;25:192-205. doi: 10.1016/0003-2697(68)90092-4 [Crossref]
- Hancock J, Desikan R, Neill
S. Role of reactive oxygen species in cell signaling pathways. Biochem Soc Trans
2001;29(2):345-9. doi: 10.1042/bst0290345 [Crossref]
- Takimoto
E, Kass DA. Role of oxidative stress in cardiac
hypertrophy and remodeling. Hypertension 2007;49(2):241-8. doi: 10.1161/01.hyp.0000254415.31362.a7 [Crossref]
- Biary N, Xie C, Kauffman J, Akar FG. Biophysical
properties and functional consequences of reactive oxygen species (ROS) induced
ROS release in intact myocardium. J
Physiol 2011;589(21):5167-79. doi: 10.1113/jphysiol.2011.214239 [Crossref]
- Khouri
EM, Gregg DE, Rayford CR. Effect of exercise on cardiac
output, left coronary flow and myocardial metabolism in the unanesthetized dog.
Circ Res 1965;17(5):427-37. doi: 10.1161/01.res.17.5.427 [Crossref]
- Frankiewicz-Jóźko A,
Faff J, Sieradzan-Gabelska B. Changes in
concentrations of tissue free radical marker and serum creatine kinase during
the post-exercise period in rats. Eur J Appl Physiol
1996;74(5):470-4. doi: 10.1007/s004210050101 [Crossref]
- Stanojevic D, Jakovljevic V, Barudzic N, Zivkovic V, Srejovic I, Ilic KP,
et al. Overtraining does not induce oxidative stress and inflammation in blood
and heart of rats. Physiol Res 2016;65(1). doi: 10.33549/physiolres.933058 [Crossref]
- Gul M, Demircan B, Taysi S, Oztasan N, Gumustekin K, Siktar E, et al.
Effects of endurance training and acute exhaustive exercise on antioxidant
defense mechanisms in rat heart. Comp Biochem Physiol A Mol Integr
Physiol 2006;143(2):239-45. doi: 10.1016/j.cbpa.2005.12.001 [Crossref]
- Dickinson DA, Forman HJ. Cellular glutathione and
thiols metabolism. Biochem Pharmacol
2002;64(5-6):1019-26. doi: 10.1016/s0006-2952(02)01172-3 [Crossref]
- Korivi M,
Hou C-W, Huang C-Y, Lee S-D, Hsu M-F, Yu S-H, et al. Ginsenoside-Rg1 protects
the liver against exhaustive exercise-induced oxidative stress in rats. Evid
Based Complement Alternat Med 2012;2012. doi: 10.1155/2012/932165 [Crossref]
- Khanna S, Atalay M,
Laaksonen DE, Gul M, Roy S, Sen CK. α-Lipoic acid supplementation: tissue
glutathione homeostasis at rest and after exercise. J Appl Physiol
1999;86(4):1191-6. doi: 10.1152/jappl.1999.86.4.1191 [Crossref]
- Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MM. The potential protective role of alpha-lipoic
acid against acetaminophen-induced hepatic and renal damage. Toxicology
2008;243(3):261-70. doi: 10.1016/j.tox.2007.10.010 [Crossref]
- Maritim A, Sanders R, Watkins Iii J. Effects of α-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem 2003;14(5):288-94. doi: 10.1016/s0955-2863(03)00036-6 [Crossref]
- Neubauer O, Koenig D, Kern N, Nics
L, Wagner K-H. No indications of persistent oxidative stress in response to an
ironman triathlon. Med Sci Sports Exerc 2008;40(12):2119-28. doi: 10.1016/s0162-0908(09)79565-0 [Crossref]
- Meihua S.
The time effect of DNA damage and oxidative stress on mice liver cells induced
by exercise fatigue. In: Education Management, Education Theory and Education
Application. Springer 2011:661-7. doi: 10.1007/978-3-642-24772-9-96 [Crossref]
- Gonçalves
AC, Rodrigues LR, Terra MP, Sasaki JE, Portari GV. Exercício aeróbio exaustivo aumenta o estresse
oxidativo em corredores fundistas treinados. Rev Bras Prescrição Fisiol Exerc 2019;13(83):493-500.
- Petry CD, EatonMA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr 1992;121(1):109-114. doi: 10.1016/S0022-3476(05)82554-5 [Crossref]
- Bustamante J, Lodge JK, Marcocci L, Tritschler HJ, Packer L, Rihn BH. α-Lipoic acid in liver metabolism and disease. Free Radic Biol Medic 1998;24(6):1023-39. doi: 10.1016/S0891-5849(97)00371-7 [Crossref]
- Navarro A, Gomez C, López-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice
aging: survival, behavior, oxidative stress, and mitochondrial electron
transfer. Am J Physiol-Regul Integr
Comp Physiol 2004;286(3):R505-11. doi: 10.1152/ajpregu.00208.2003 [Crossref]
- Taysi S, Oztasan N, Efe H, Polat M, Gumustekin K, Siktar E, et al. Endurance training attenuates the oxidative stress due to acute exhaustive exercise in rat liver. Acta Physiol Hung 2008;95(4):337-47. doi: 10.1556/aphysiol.95.2008.4.2 [Crossref]