Rev Bras Fisiol Exerc 2021;20(2):149-61
doi: 10.33233/rbfex.v20i2.4300
ORIGINAL ARTICLE
Plasmatic and urinary analysis of muscle damage
biomarkers in professional futsal athletes
Análise
sérica e urinária de biomarcadores de dano muscular em atletas profissionais de
futsal
Luciano
de Oliveira Siqueira1, Maria Eduarda Kegler
Ramos1, Daniel Schwarzbach2, Lucas Marostica3,
Jose Claudio Fonseca Moreira4
1Universidade de Passo Fundo, Passo
Fundo, RS, Brazil
2Pharmaceutical Professional, Erechim,
RS, Brazil
3Universidade Federal de Santa Catarina,
Florianópolis, SC, Brazil
4Universidade Federal do Rio Grande do
Sul, Porto Alegre, RS, Brazil
Received:
June 25, 2020; Accepted February 17, 2021.
Correspondence: Luciano de Oliveira Siqueira, Br 285 km, 171, 99052-900
Passo Fundo RS. luciano@upf.br
Luciano de Oliveira Siqueira: luciano@upf.br,
Maria Eduarda Kegler Ramos:
182278@upf.br
Daniel Schwarzbach: danibach11@yahoo.com.br
Lucas Marostica:
lucasmarostica@yahoo.com.br
Jose Claudio Fonseca Moreira: 00006866@ufrgs.br
Abstract
Introduction: In the view of
metabolic changes resulting from sport and the consequences of overcoming individual
limits in elite team sports, it is necessary to understand biochemically the
phenomenon that occurs in this modality to minimize damage, improve athletic performance and provide protocols more suitable for every
sport and individual. Aim: The present study aimed to analyze the
metabolism of professional athletes during training by determining blood and
urinary biomarkers of proteolysis, lipolysis, hemolysis, and muscle
microtrauma. Methods: The evaluation was carried out with 12 athletes
participating in the Gold series of a futsal league. All were submitted to a
standard training protocol and blood and urine samples were collected at rest
and 15 minutes after the training session. Results: The statistical
analysis of the results showed a significant increase (p < 0.05) in the
relative (cells /%) and absolute (cells/µL) counts of neutrophils, as well as
in the serum concentration of uric acid, total cholesterol and HDL fractions,
ALT, LDH and CK-MM. On the other hand, it also showed a statistically
significant decrease (p < 0.05) in the concentration of magnesium, glucose,
urinary urea, relative lymphocyte count, absolute monocyte, and eosinophil
count (cells /% and cells/µL). Conclusion: The results obtained here
allowed us to conclude that biochemical understanding can minimize muscle
damage, improve athletic performance, and offer the development of more
appropriate protocols for each sport.
Keywords: biochemistry; sports medicine;
athletic performance.
Resumo
Introdução: Diante das alterações metabólicas
advindas do esporte e as consequências da superação dos limites individuais nos
esportes coletivos de elite, faz-se necessária a compreensão bioquímica dos
fenômenos ocorridos nessa modalidade a fim de minimizar danos, aperfeiçoar o
desempenho atlético e fornecer protocolos mais adequados para cada esporte e
indivíduo. Objetivo: O presente estudo objetivou analisar o metabolismo
de atletas profissionais durante o treinamento mediante determinação de
biomarcadores sanguíneos e urinários de proteólise, lipólise, hemólise e microtrauma muscular. Métodos: A avaliação foi
realizada com 12 atletas que participam na série Ouro da liga de futsal. Todos
foram submetidos a um protocolo padrão de treinamento e amostras de sangue e
urina foram coletadas em repouso e 15 minutos após a sessão de treinamento. Resultados:
A análise estatística dos resultados mostra um aumento significativo (p <
0,05) nas contagens relativa (células/ %) e absoluta (células/µL) de
neutrófilos, bem como na concentração sérica de ácido úrico, colesterol total e
frações HDL, ALT, LDH e CK-MM. Por outro lado, apresentaram uma diminuição
estatisticamente significativa (p < 0,05) na concentração de magnésio,
glicose, ureia urinária, contagem relativa de linfócitos, contagem absoluta de
monócitos e eosinófilos (células /% e células/µL). Conclusão: Os
resultados aqui obtidos permitem concluir que a compreensão bioquímica pode
minimizar dano muscular, aperfeiçoar o desempenho atlético e oferecer formação
de protocolos mais adequados para cada esporte.
Palavras-chave: bioquímica; medicina esportiva;
desempenho atlético.
Introduction
According to
World Health Organization (WHO) physical activity promotes mental (such as
reducing stress [1]) and physical health. In that sense, practicing regularly
can promote several benefits [1,2]. The High Performance
Sports (HPS) is an elite sports practice in which the athlete dedicates a
significant amount of training time to reach the maximum performance and
results, being subjected to heavy training and stress rounds [3]. Therefore, in
the view of alterations from metabolic bodily activities in sports and its
relevance when combined with the correct practice it is vital to comprehend the
biochemistry of processes that occur during elite collective sports aiming to
minimize muscular damage, improve athletic performance and provide proper protocols
to each sport and individual.
The HPS
practice, if based only in results, can be harmful since the athletes are
submitted to heavy loads with small recovery periods with no respect for its
individualities and striving to overcome its limits [4]. In intense physical
exercise, a metabolic stress is created due to an increased energetic demand
with a more substantial synthesis of oxidants. The high muscular activity
results in an increase of oxygen reactive species that exceed the body’s
antioxidant capacity therefore promoting an oxidation that can generate
muscular damage with reverse consequences such as strength loss and decreased
athletic performance [5,6].
Plasmatic and
urinary analysis of biochemical markers can contribute to finding and
monitoring this overload since HPS can increase the plasmatic rate of muscle
damage marker enzymes such as creatine kinase (CK) and lactate dehydrogenase
(LDH); as well as signaling the tightening of muscle fibers, injuries, inflammations and adaptive micro-traumas [7]. In this
context, futsal is a sport with predominant demand of the aerobic system,
however, it consists of high intensity actions that demands great physical, tactical and technical level from its players with the
supportive use of the anaerobic metabolism [8,9]. Thus, the occurrence of
traumatic events or injuries is common during matches [10].
Sports medicine
has advanced in the last decades especially for its emphasis in health,
improving quality of life, injury prevention and maximization of performance of
training for professional or amateur athletes [11]. Through a specialized
treatment and physiological and biochemical comprehension of this athlete’s
routines, a multi-professional team evaluate and manages practice with
specialized diets, adjusting its intensity and duration. This can produce
positive results when preventing, treating, and rehabilitating injuries besides
maximizing the athlete’s performance. That way, the athlete can reach more
competitive results that, when combined with a healthy lifestyle, can
contribute to its adaptation.
Based on such
premises, the present study aimed to analyze the metabolism and biochemical
effects of futsal sport in professional athletes during training by determining
plasmatic and urinary biomarkers of proteolysis, lipolysis, hemolysis, and
muscular micro-trauma.
Methods
Design
This is a
cross-sectional, prospective, and experimental study of a futsal team
participating in the National Futsal League.
Sample
Twelve male athletes members of a futsal team participating in the
National Futsal League participated in this study. The individuals included in
the experiment were starting and reserve athletes with no history of injuries
within the past year; professional athletes for over 2 years; training
regularly at the team for over 1 year; with a diet controlled and oriented by a
nutritionist. Athletes removed because of injuries; using medication; with
recent injury and with chronic illness such as diabetes and high blood pressure
were excluded.
A convenience
sample was used since the sample characteristics were very specific (athletes
from a futsal team) and homogeneous (elite participating in the National Futsal
League).
Ethical aspects
This research
was developed according to declarations and guidelines regarding research with
human beings: the Nuremberg Code, Helsinki Declaration
and the National Health Council resolution nº 466/12. This research was also
approved and regulated by the Universidade de Passo Fundo Ethics Committee, with an approved CAAE
18778119.5.0000.5342, opinion n° 3.688.601.
Environmental conditions
The training
session was performed during the morning at the sports court with wooden floor
(team’s headquarters) and lasted approximately 90 minutes. The tests were
conducted with 15ºC of room temperature, 88% of relative air humidity (rainy
day) and 783 meters above sea level. The athletes were dressed in light
training clothes (dry fit).
Training protocol
The training
protocol included approximately 30 minutes of physical activity subdivided in 3
phases:
* 10 minutes of light run;
* 10 minutes alternating between 10 seconds of fast
run and 20 seconds of light run;
* 10 minutes alternating between 30 seconds of light
fast run and 90 seconds of light run.
After aerobic training,
the athletes performed 30 minutes of lower limbs exercises composed of three
sets with 12 repetitions each and 45 seconds for recovery between machines.
Blood samples
Blood samples
were collected with antisepsis of the athlete’s antecubital fossa while resting
and 15 minutes after the end of the training session. A 2 mL sample was stored
in flasks with 2 mg/mL of EDTA (ethylenediaminetetraacetic acid) for blood
analysis. A full hemogram with platelets was carried out by impedance ABX
micros 60® (ABX diagnostics, Montpellier, France) electronic counting of cells.
A differential count of leukocytes was performed by microscopic analysis of 200
cells (Nikon Eclipse 600 ®, Nikon Corporate Instruments, Japan) in blood sheets
colored with the Romanowsky (Merck®) technique. Differential analysis of cellular
strains was expressed by relative (cells/%) and absolute (cells/µL) count.
To obtain the
serum, the rest of the sample (8 mL) was stored in an anticoagulant test-tube.
Then, the sample was centrifuged with 1500 rpm for 15 minutes. The serum was
extracted and placed in test-tubes pre-treated with nitric acid 30% for 24
hours and rinsed five times with double-distilled water in order to
spectrophotometrically measure biochemical parameters such as calcium,
phosphorus, magnesium, glucose, uric acid, creatinine, urea, total proteins,
aminotransferases (ALT/AST), alkaline phosphatase, lactate dehydrogenase (LDL),
total creatine kinase, MB creatine kinase, triglycerides, total cholesterol and
fractions using enzymatic methods in commercial kits following the manufacturer
norms (Labtest® Diagnostica
AS - Belo Horizonte, Brazil) with a semiautomatic equipment from Labquest® (Labtest® Diagnóstica AS, Belo Horizonte, Brazil). The sodium,
potassium and chlorides were measured by the Medica EasyLite®
(Medica Corporate Profile, Bedford, Massachussetts
EUA) technique of selective ion electrode.
Urine samples
A sample of
approximately 50 mL of urine, medium stream with previous rinsing of the
genitals was collected in universal collector bottles of resting athletes and
15 minutes after the training session. The samples were collected in standard
bottles that were transferred from the collection site to the laboratory under
controlled temperature. Then, they were immediately processed and analyzed with
a microscope as recommended by the ABNT-CB 36 (in vitro). After, samples were
centrifuged with 1800rpm for 10 minutes and 1 mL of supernatant was reaped for
later biochemical analysis of total urinary proteins, uric acid, creatinine,
and urea (Labtest® Diagnóstica
S.A.).
Physical-chemical
analysis was performed by visual observation of the urine aspects and color and
density was measured by hand refractometer (LF® Equipamentos
hospitalares, São Paulo, Brazil).
Chemical
analysis was carried out with polyelectrolytes testing stripes (ComburTest Dade-Behring®) to determine blood proteins,
glucose, bilirubin nitrite, pH, ketones, leukocytes, and urobilinogen within
the non-centrifuged sample.
Microscopic
analysis examined the urinary sediment searching for epithelial cells,
crystals, leukocytes, erythrocytes, bacteria, mucus wiring.
Statistical analysis
Results were
transposed to a spreadsheet for analysis of central tendency (mean) and
dispersion (standard deviation) measures. Data were analyzed for normality by
the Kolmogorof-Smirnov test. Variables with Gaussian
distribution were analyzed statistically by comparing the means with a “t” test
for paired samples (parametric data). For variables without regular
distribution, the Wilcoxon-Mann-Whitney test was applied (non-parametric data),
considering p < 0.05 as the minimum level of significance.
Results
The mean age was
25.5 ± 4.8 years; with 170 ± 4 cm height; 74.6 ± 7.6 kg body weight; and BMI of
24.2 ± 1.9 kg/m². All subjects were training for more than two years using
athletic shoes provided by the team’s sponsoring company and were in great
physical condition. Besides, all subjects were not using any medication or
supplements that could have interfered in the analysis and outcomes of the
study. All athletes were resting within 48h prior to the training session.
Table I – Analysis of the acute effects
of training in biochemical and blood parameters of athletes while resting and
15 minutes after the training session
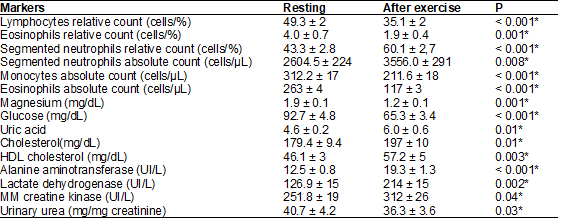
Results are presented
as ± DP; *p < 0,05 regarding the resting athletes by analysis of parametric
data with “t” test for paired samples
Table I
presented the analysis of acute training effects in biochemical and blood
parameters of athletes while resting and 15 minutes after the training session.
Results demonstrated a statistically significant increase (p < 0,005) in
relative (cells/%) and absolute (cells/µL) count for segmented neutrophils,
uric acid plasmatic concentration, total cholesterol, HDL cholesterol, ALT,
LDH, CK-MM, that seems to be associated with a statistically significant
decrease (p < 0.05) in concentrations of magnesium, glucose and urinary
urea, lymphocytes relative count, monocytes absolute count and eosinophils
relative and absolute counts.
However, in
parameters such as: red blood cells, hemoglobin, hematocrit, MCH, MCV, MCHC,
platelets, proteins, urea and plasmatic and urinary creatinine, uric acid, AST,
calcium and plasmatic chlorine, LDL and VLDL cholesterol, triglycerides,
alkaline phosphatase, phosphorus, total creatine kinase and MB fraction, iron
and potassium counts did not present a significant difference (p > 0,05)
between resting and after exercise (data not shown).
Table II – Analysis of the acute effects
of training in physical and chemical parameters of urine in athletes while
resting and 15 minutes after the training session
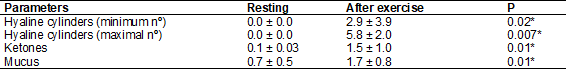
Results are presented
as ± DP; *p < 0,05 regarding the resting athletes by the
Wilcoxon-Mann-Whitney test for non-parametric data
On the other
hand, Table II analyzed the effects of acute training in physical and chemical
parameters of urine in athletes while resting and 15 minutes after the training
session. Results showed a statistically significant increase (p < 0,05) in
the amount of mucus wiring and hyaline cylinders, indicating a possible
reduction in urinary flow due to the loss of renal water induced by sweating,
besides an increase in urinary ketones indicating a lipolytic scenario.
In contrast,
variables of physical-chemical analysis of urine, such as: color, aspect
(turbidity), density, leukocyte reaction, urobiligenin,
pH, red blood cells and hemoglobin, bilirubin, glucose, epithelial cells
microscopic analysis, amorphous urates, uric acid crystals and calcium oxalate
crystals; did not show any significant difference (p < 0,05) (data not
shown).
Discussion
Sports practice
generates several benefits when conducted with planning, adapting the
individual’s metabolism to physiological and biochemical alterations to each
type, intensity, and duration of training. The lack of adequacy can promote
damage to the organism, such as increasing the susceptibility for muscular
injuries, inflammatory diseases, reduction of antioxidant capacity, loss of
muscular mass and strength, fatigue and decreased athletic performance [12,13,14].
The red blood
cells analysis from hematologic profile did not present any significant
difference in post-training when compared to resting for the following
parameters: hemoglobin, erithrometry, hematocrit,
MHC, MCV, MCHC and plasmatic platelets in this investigation. Those findings
are supported by Bezerra [12], that evaluated
biochemical parameters of 42 professional male soccer athletes prior, after,
24, 48 and 72 hours after the match, and did not find significant differences
in such parameters. This is a basic analysis for diagnosing anemia being an
indicator for oxygenation and hydration in sports medicine. In that sense,
erythropoietin, for example, is a hormone that raises the red series numbers,
improving the oxidant capacity – its exogenous implementation is considered
doping [15]. The hematocrit measures the relationship between plasma/cell and
is roughly affected by hydration. Therefore, rises in the hematocrit value
seems to be relate to dehydration and hemoconcentration [16]. The results
analysis did not show any significant difference regarding the mild room temperature
(15ºC) in training day and adequate hydration.
Also
within Bezerra [12] study, its results demonstrated
that in the collection performed 24h after practice there was a small reduction
of hematocrits, hemoglobin and erythrocytes that may be justified by
hemodilution [16] and mechanical stress that can accentuate hemolysis. The iron
indexes, an essential ion for hemoglobin synthesis and oxygen transportation,
remained with no significant difference in this study.
Table I presents
a statistically significant increase in relative and absolute counts of
segmented neutrophils combined to a significant reduction in relative count of
lymphocytes and eosinophils and absolute count of eosinophils and monocytes.
Physical exercise activates the hypothalamus–pituitary –adrenal axis [17]
inducing an inflammatory response characterized by leukocytosis that can be
caused, among other factors, by the increase of neutrophils whom
have as its function to remove undesirable elements related to tissue injuries
by phagocytosis [12,18]. Blood flow has a laminar characteristic, its viscosity
and cellular elements that generates friction with the blood vessel walls
generating a shear force (Figure 1). Thus, smaller elements as red blood cells,
run in an axial way at the center of the flow surrounded by a plasma stream
where circulation leukocytes run. At the edge, marginal leukocytes are located
due to the plasma’s viscosity and shear force that makes them dislocate with a
lower speed that the circulation pool. The catecholamines elevation promotes
hemodynamic changes inducing the marginal leukocytes pool inside the blood
vessel to migrate combined with the leukocytes in the middle of the vessel,
justifying the development of a neutrophil leukocytosis associated with a
reduction of monocytes, lymphocytes and eosinophils reported here [19].
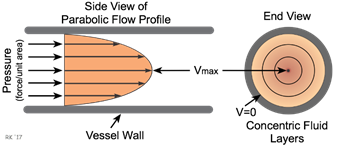
Source:
Cardiovascular Physiology Concepts [34]
Figure 1 – Laminar blood flow
According to
Table I, the protein, uric acid and creatinine urinary concentration analysis
did not show any significant difference after practice. This finding responds
to the study of Neto [13] that reported that the HIE (High Intensity Exercise)
induced a scenario of proteinuria, cylinders, and red blood cells within the
urine samples. This increase was attributed to a possible vasoconstriction of
the renal circulation altering the hydrostatic pressure at the glomerulus also
promoting the passing of proteins and red blood cells through the glomerular
basal membrane, especially when associated with a high protein diet. It can be
concluded that the experimental protocol applied in this study was not intense
enough to promote the alteration indicated above.
On the other
hand, these results exposed a statistically significant reduction of urinary
urea levels combined with an increase of the amount of mucus and urinary
cylinders. Sports practice promotes an increase of non-renal hydric loss
through sweating that can reduce the bodily hydric content, volemia
and urinary debit promoting the gelation of Tamm Horsfall proteins within renal
tubules inducing the formation of mucus and cylinders due to hydric loss [20].
The analysis of
other urinary parameters: color, aspect (turbidity), density, leukocyte
reaction, urobilinogen, pH, red blood cells and hemoglobin, bilirubin, glucose,
epithelial cells microscopic analysis, amorphous urates, uric acid crystals and
calcium oxalate crystals, did not evidence any significant difference. Those
findings support the ones found by Neto [13] that also did not find any
significant alterations regarding such variables after HIE. On the other hand,
the proposed training promoted a statistically significant elevation in the
number of urinary ketones that indicate a lipolytic scenario justified by the
habit common among most athletes of training while fasting in the morning.
Among
nitrogenous compounds, there was a significant increase in uric acid plasmatic
concentration combined with a reduction of urinary urea. Physical activity
promotes an increase in the metabolism of purine bases [21], that is biotransformed in uric acid via xanthine oxidase [22], that
may act as an antioxidant. Hyperuricemia occurs when there is an increase of
metabolism and/or reduction of the uric acid elimination and can happen in
disorders in homeostasis of sodium and water [21]. Plasmatic urea can result
from proteolysis with a major renal excretion. The results analysis showed a
decrease in urea’s renal elimination; however its
plasmatic concentration did not suffer significant alterations, indicating that
the proteolysis scenario was incipient and that the urinary reduction may have
occurred due to a volemic reduction scenario,
especially when analyzed with the appearance of mucus and cylinders [23].
Regarding the lipid
profile, Table I shows that the total cholesterol and HDL cholesterol had a
significant increase after practice, while LDL cholesterol, VLDL and
triglycerides did not. Our results correspond with existing literature, since
practicing sports seems to improve the lipid profile, enhancing the muscular
tissue capacity for using fatty acids and raising the activity of the
lipoprotein lipase reducing the triglycerides levels and generating a balance
between HDL and LDL cholesterol, being considered the first factor of
protection against cardiovascular diseases [24,25].
Córdova [26]
defined magnesium (Mg) as an essential mineral relevant for the energetic
metabolism and for the muscular contraction and relaxation function, considered
a co-factor of enzymatic reactions involved in anabolic and catabolic processes
that affects the muscular performance [26,27]. In the present study, is was verified a statistically significant reduction in
post-practice magnesium levels, as corroborated by Castro [14], that confirms
the high loss of magnesium within blood and urine through urine and sweat in
EAR. This reduction of magnesium levels associated with an elevation of
creatine kinase (CK-mm) can lead to an understanding that the training protocol
applied was characterized by a micro-injuries
scenario. Besides, since Mg is a glycolytic route enzyme co-factor, there was
also evidence that appropriate levels of Mg would indicate a fatigue scenario
when associated to glycemic reduction and reports of it after training [27].
Muscular damage
can be detected by analyzing the activity of muscular enzymes such as creatine
kinase (total and fraction mm CK total), lactate dehydrogenase (LDH) or
aspartate aminotransferase (AST) [28]. CK and LDH are heavy chain fragments
from myosin and are related to muscular tissue injuries [29]. They are found in
cell’s cytosol and do not have the capacity to go through the sarcoplasmic
barrier [28,29]. Total CK is an indirect marker of tissue damage that catalyzes
the creatine transformation in phosphocreatine from adenosine triphosphate
(ATP), used to detect muscular fatigue and overload. LDH is a marker of
musculoskeletal injuries by muscular fibers disruption and can be understood as
a depletion of strength and inflammation. Combined monitoring of CK and LDH
levels shows the degree of metabolic adaptation of skeletal muscles [30].
Results analysis
showed a statistically significant raise in the activity of the three enzymes.
Studies from Barranco [28], Bezerra
[12] and Ferreira [31] reported increasing in plasmatic concentration of CK,
LDH and AST enzymes in athletes after futsal, soccer and cycling practices,
respectively. The raise of muscular enzymes can signal structural alterations
within muscular fibers indicating that practice is causing overload with
resulting loss of muscular and athletic performance [31]. In the present study,
there was a significant increase in plasmatic activity of CK-MM and LDH, while
there was no significant difference in total creatine kinase, AST levels and
CK-MB fraction. Such biochemical parameters can be used as strategy planning
for muscular recovery of players as well as in the reduction of damages and
consequent improved muscular performance.
Aminotransferases
are hepatic enzymes that can suffer alterations in its levels after physical
exercise, indicating hepatic, renal, cardiac, or muscular injuries [32]. AST
(aspartate aminotrasnferase) has the higher
participation in muscular lysis and elevation index related to ALT (alanine
aminotransferase) and can triple after intense training. ALT is present in a
higher quantity within hepatocytes; thus, its raise has a higher probability of
indicating hepatic pathology or happen due to an increase in internal body
temperature [31]. They increase due to excessive muscular tension, by damage or
deterioration of skeletal muscles. In our study, AST levels did not show any
significant differences at resting or after practice. On the other hand, ALT
levels raised significantly. When there is lack of glycogen, the liver needs to
produce glucose through other sources, promoting an increase in hepatic
glycogenesis that uses lactate and amino acids as substrate. Therefore, due to
protein catabolism, this enzymes activity can enhance [30].
Bone metabolism
affects athletic performance as well as executing physical exercises and has an
important role in bone mineralization, helping to maintain skeletal integrity
and reduce fracture risk [33]. However, the analysis of alkaline phosphatase,
calcium and phosphorus activity did not show any significant difference in this
study.
The present
investigation has some limitations, as the small sample of only 12 male
athletes that do not represent conclusive reference values. Another limitation
was that, although an anamnese regarding the use of
medication and supplements, there was no diet follow-up in the days prior to
the study. On the other hand, the highlight and innovation in our study was
that no literature was found that presented and evaluated all the parameters
combined. Besides, it is unique for its homogeneity of the futsal athletes group since it was the world futsal champions, with
high physical preparation and athletic performance. Moreover, our results allow
an understanding over the body biochemical functioning during sports practice
and has characteristics and descriptive data with little variance that can be
take into consideration for future research. Furthermore, this analysis was
carried out with adapted athletes undergoing high performance training for over
2 years.
Lastly, we
recognize the need to comprehend the analysis of biochemical markers
corresponding to proteolysis, lipolysis, hemolysis, and muscular damage during
training sessions to prevent the low of athletic and muscular performance, as
well as physical capacities, enabling the achievement of good results.
Conclusion
Results obtained
in this research allow us to conclude that the proposed training protocol
induced a lipolytic scenario caused by the spoliation of carbohydrates from a
low supply (fasting) e glycolytic activity from practice. However, it did not
present any relevant laboratory signals of proteolysis. Moreover, the raise of
enzymatic biomarkers characterized an adaptive micro-injury with no signs of
muscular damage/injury due to its increase of 25% (CK-mm) and 40% (LDH).
Lastly, blood alterations presented in the hemogram indicated a physiological
reflex from hypothalamo-pituitary-adrenal axis
characterized by neutrophil leukocytosis with lymphocytopenia. Thus, the
experimental training protocol proposed was satisfatory
from a biochemical point of view since the metabolic adaptations characterize
an adaptation to training with no laboratory signs of damage.
Acknowledgements
We
appreciate the management, technical team and especially the C.E.R Atlântico
Erechim Futsal team that volunteered for this study.
Potential conflict of interest
No conflicts of interest
with relevant potential for this article was reported.
Financing
Own financing
Authors contribution
Research conception and design: Siqueira LO; Data collection:
Siqueira LO, Schwarzbach D, Marostica LL; Data analysis and interpretation:
Siqueira LO, Moreira JCF; Statistical analysis: Siqueira LO; Financing
acquisition: Siqueira LO; Manuscript writing: Siqueira LO, Ramos MEK; Manuscript
critical review as for relevant intelectual content: Siqueira LO, Ramos MEK,
Moreira JCF.
References
- Oliveira VADS, Sinésio IYC, Cabral PUL,
Cortez ACL, Meneses YPSF. Associação de atividade física e o estresse em
pré-vestibulandos. Rev Bras
Fisiol Exerc 2019;18(1):9-16.
doi: 10.33233/rbfe.v18i1.2875 [Crossref]
- Organização Mundial da Saúde 2020
[Internet]. Physical. [citado 2021 Jan 15]. Available from:
https://www.who.int/health-topics/physical-activity#tab=tab_1
- Soares TC, Silva LAA, Norões ARL, Medeiros
SRA, Cavalcante RMS. Efeitos da suplementação de glutamina em atletas de alto
rendimento: uma revisão de literatura. Rev Brasileira
de Nutrição Esportiva [Internet]. 2019;13(77):17-6. [citado 2021 Jan 15]. Available from:
http://www.rbne.com.br/index.php/rbne/article/view/1215
- Ministério da cidadania [Internet]. AFEs,
Desenvolvimento humano e esporte de alto rendimento. [citado 2021 Jan 15].
Available from: http://www.esporte.gov.br/snear/departamentos.jsp
- Coqueiro AY, Mata Godois
AM, Raizel R, Tirapegui J.
Creatina como antioxidante em estados metabólicos envolvendo estresse
oxidativo. Revista Brasileira de Prescrição e Fisiologia do Exercicíco
[Internet] 2017;11(64):128-37. [citado 2021 Jan 15]. Available from:
http://www.rbpfex.com.br/index.php/rbpfex/article/view/1090
- Steinbacher P, Eckl P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015;5(2):356-77. doi: 10.3390/biom5020356 [Crossref]
- Chielle EO, Maziero JS. Efeito do exercício físico intenso nas concentrações sérica, salivar e urinária de marcadores de lesão músculo esquelética. Evidência - Ciência e Biotecnol 2018;18(1):41-58. doi: 10.18593/eba.v18i1.16666 [Crossref]
- Fikri S, Pradhila F, Dirganta D. Biomechanical analysis with optimal combination by using foot and distance when the futsal player passing the ball against the accuracy of the target. Br J Sports Med. 2016;50(22):e4.13-e4. doi: 10.1136/bjsports-2016-096952.20 [Crossref]
- Pereira-Neto E, Alves RC, Souza Júnior TP, Brandão LHA, Silva-Grigoletto ME, Almeida MB. Efeito agudo do treinamento de força com restrição de fluxo sanguíneo sobre demanda metabólica de lactato em jovens futebolistas. Rev Bras Fisiol Exerc 2019;18(3):136. doi: 10.33233/rbfe.v18i3.3239 [Crossref]
- Tavana B, Nourian R, Memari AH, Noormohammadpour P, Kordi R. O-33 Futsal and injuries among recreational sport participants: a prospective field study. Br J Sports Med 2016;50(1):A19.1-A19. doi: 10.1136/bjsports-2016-097120.33 [Crossref]
- Silva TT. Questões éticas na prática da
medicina do esporte na contemporaneidade. Rev
Bioética 2019;27(1):62-6. doi: 10.1590/1983-80422019271287 [Crossref]
- Bezerra JA, Farias NO, Melo SVA, Silva RPM, Castro ACM, Martins FSB, et al. Respostas de indicadores fisiológicos a um jogo de futebol. Rev Bras Med do Esporte 2016;22(3):200-5. doi: 10.1590/1517-869220162203137068 [Crossref]
- Neto OPM, Carvalho AS, Nicolau LS, Costa AC, Dias GPS. Análise da proteinúria após exercício físico intenso. Rev Bras Anal Clin 2017;49(3):256-62. doi: 10.21877/2448-3877.201700564 [Crossref]
- Castro KR, Dantas MP, Teixeira R V, Santos
GC, Neto PA, Ururahy MG, et al. Relação de magnésio
sérico e capacidade de sprints repetidos em crianças. Rev
Motricidade 2019;15(1)23-8.
- Bueno C. Competições de alto risco. Ciência e Cultura 2017;69(2):22-4. doi: 10.21800/2317-66602017000200009 [Crossref]
- Marques GC, Ferre-Douza
V. Análise do perfil hematológico de praticantes de atividade física para
hipertrofia muscular, usuários ou não de suplementos e esteróides
anabolizantes. FOCO: Caderno de Estudos e Pesquisa [Internet] 2017;13:54-70. [citado 2021 Jan 15]. Available
from:
http://www.revistafoco.inf.br/index.php/FocoFimi/article/view/497
- Duclos M, Tabarin A. Exercise and the hypothalamo-pituitary-adrenal axis. Front Horm Res 2016;47:12–26. doi: 10.1159/000445149 [Crossref]
- Lima FD, Oliveira RJ, Albuquerque RC, Correia ALM, Dantas RAE, Mota MR. Respostas hematológica agudas ao teste incremental máximo em esteira. Motricidade 2016;12(3):39-44. doi: 10.6063/motricidade.6327 [Crossref]
- Dias R, Baganha RJ, Cieslak F, Krinski K, Camarço NF, Verlengia R, et al. Parâmetros imunológicos e infecções do trato respiratório superior em atletas de esportes coletivos. Rev Bras Med do Esporte 2017;23(1):66–72. doi: 10.1590/1517-869220172301149299 [Crossref]
- Morales JV, Guimarães J, Barros E.
Proteinúria: avaliação clínica e laboratorial. Clin Biomed Res [Internet] 2006;16(3):95-101. [citado 2021 Jan 15]. Available from: https://seer.ufrgs.br/hcpa/article/view/99823
- Wolyniec W, Ratkowski W, Kasprowicz
K, Malgorzewickz S, Aleksandrowickz
E, Witek K, et al. Changes in electrolytes and uric
acid excretion during and after a 100 km run. J Biol Regul
Homeost Agents [Internet] 2018;5:1205-10.
[citado 2021 Jan 15]. Available from:
https://pubmed.ncbi.nlm.nih.gov/30334414/
- Ndrepepa G. Uric acid and cardiovascular disease. Clinica Chim Acta 2018;484:150-63. doi: 10.1016/j.cca.2018.05.046 [Crossref]
- Silva ASR, Santhiago
V, Papoti M, Gobatto CA. Comportamento das
concentrações séricas e urinárias de creatinina e uréia
ao longo de uma periodização desenvolvida em futebolistas profissionais:
Relações com a taxa de filtração glomerular. Rev Bras Med Esporte 2006;12(6):327-32. doi:
10.1590/S1517-86922006000600006 [Crossref]
- Paula A, Ferreira S, Picolli T, Bordin A, Rech A, Poeta J, et al. Baixos níveis de atividade física estão associados a prejuízos no perfil lipídico e aumento do percentual de gordura de indivíduos idosos. Rev Bras Ciênc Mov 2015;23(3):135-42. doi: 10.18511/0103-1716/rbcm.v23n3p135-142 [Crossref]
- Ghafouri K, Cooney J, Bedford DK, Wilson J, Caslake MJ, Gill JMR. Moderate exercise increases affinity of large very low-density lipoproteins for hydrolysis by lipoprotein lipase. J Clin Endocrinol Metab 2015;100(6):2205-13. doi: 10.1210/jc.2015-1196 [Crossref]
- Córdova A, Mielgo-Ayuso J, Fernandez-Lázaro D, Roche E, Caballero-García A. Impact of magnesium supplementation in muscle damage of professional cyclists competing in a stage race. Nutrients 2019;11(8):1927. doi: 10.3390/nu11081927 [Crossref]
- Zhang Y, Xun P, Wang R, Mao L, He K. Can magnesium enhance exercise performance? Nutrients 2017;9(9):946. doi: 10.3390/%2Fnu9090946 [Crossref]
- Barranco T, Tvarijonaviciute A, Tecles F, Carrillo JM, Sánchez-Resalt C, Jimenez-Reyes P, et al. Changes in creatine kinase, lactate dehydrogenase and aspartate aminotransferase in saliva samples after an intense exercise: A pilot study. J Sports Med Phys Fitness 2018;85(6):910-6. doi: 10.23736/s0022-4707.17.07214-0 [Crossref]
- Callegari GA, Novaes JS, Neto GR, Dias I, Garrido ND, Dani C. Creatine kinase and lactate dehydrogenase responses after different resistance and aerobic exercise protocols. J Hum Kinet 2017;58(1):65-72. doi: 10.1515/%2Fhukin-2017-0071 [Crossref]
- Isik O, Yildirim I, Ersoz Y, Koca HB, Dogan I, Ulutas E. Monitoring of pre-competition dehydration-induced skeletal muscle damage and inflammation levels among elite wrestlers. J Back Musculoskelet Rehabil 2018;31(3):533-540. doi: 10.3233/bmr-170955 [Crossref]
- Ferreira BE, Morel EA, Tsalikis
J, Costa CH, Mullher PDTG, Pontes ERJC. Alterações
agudas induzidas por competição de ciclismo em biomarcadores enzimáticos e
imunológicos. Revista Brasileira Prescrição e Fisiologia do Exercicío
[Internet] 2015;9(52):175-80. [citado 2021 Jan 15]. Available from:
http://www.rbpfex.com.br/index.php/rbpfex/article/view/752
- Krefta
B, Vechi C, Pinezi FG, Mezzomo TR. Monitoramento de eletrólitos,
de marcadores de danos musculares e função renal em atletas de futebol de
elite. Revista Brasileira de Nutrição Esportiva [Internet] 2018;11(68):1042-9.
[citado 2021 Jan 15]. Available from:
http://www.rbne.com.br/index.php/rbne/article/view/960
- Filippella M, Altieri B, Falchetti A, Cosso R, Cena H, Musso C, et al. Bone metabolism, bone mass and structural integrity profile in professional male football players. J Sports Med Phys Fitness 2020;60(6):912–8. doi: 10.23736/S0022-4707.20.09913-2 [Crossref]
- Cardiovascular
Physiology Concepts [Internet]. Laminar Flow; 2018. [citado
2021 Jan 15]. Available from: http://www.cvphysiology.com/Hemodynamics/H006