Rev Bras Fisiol exerc 2021;22(3);367-77
doi: 10.33233/rbfex.v20i3.4390
ORIGINAL ARTICLE
Acute metabolic and inflammatory responses in active
men undergoing postural corrective training
Respostas
metabólicas e inflamatórias agudas em homens ativos submetidos ao treinamento
corretivo postural
Carla
Nascimento dos Santos Rodrigues1, João Manoel Alves1,
Vanessa de Oliveira Furino1, Diego Adorna Marine1, Marco
Antônio de Lima1, Fernando Fabrizzi2, Ana Cláudia Garcia
de Oliveira Duarte1
1Universidade Federal de São Carlos
(UFSCar), São Carlos, SP, Brazil
2Faculdade de Filosofia, Ciências e
Letras de Penápolis (FAFIPE/FUNEPE), Penápolis, SP, Brazil
Received:
September 25, 2020; Accepted:
April 12, 2021
Correspondence: Carla Nascimento dos Santos Rodrigues, Centro de Ciências
Biológicas e da Saúde, Departamento de Educação Física e Motricidade Humana,
Laboratório de Nutrição e Metabolismo Aplicados ao Exercício, Rodovia
Washington Luiz, Km 235, 13565-905, São Carlos SP
Carla Nascimento dos Santos Rodrigues:
ufscar.carla@gmail.com
João Manoel Alves: joaomalves1792@gmail.com
Vanessa de Oliveira Furino:
vafurino@hotmail.com,
Diego Adorna Marine: diegomarine@gmail.com
Marco Antonio de Lima:
marcoantoniodelima@gmail.com
Fernando Fabrizzi:
ferfabrizzi@funepe.edu.br
Ana Cláudia Garcia de Oliveira Duarte: anaclau@ufscar.br
Abstract
Introduction: The practice
of physical exercises has become recurrent throughout life in the search of
health promotion. Among numerous methods offered by the fitness market, the
Corrective Postural Training (TCP) aims, through a gymnastics model, to
increase adherence to the practice, with a reduced risk of muscle-joint
injuries. Objective: To analyze the effects of an initial session of TCP
on lactic acidemia, blood glucose, heart rate, interleukin-6, tumor necrosis
factor-alpha and creatine kinase in healthy individuals without previous
experience with the method. Methods: 16 active men, without preexisting
diseases (28.5 ± 5.0 years; 72.16 ± 8.1 kg; 1.75 ± 0.06 m and body mass index
23.31 ± 2.1) underwent three visits to the laboratory. Two visits for data
collection and one for the TCP session. Results: The session showed
stable levels for the variables of blood glucose, creatine kinase, tumor
necrosis factor-alpha and interleukin-6. Significant differences were found for
lactic acid in the 20' (4.9 ± 1.5 mmol/L) and 30' (4.1 ± 1.6 mmol/L) minutes of
the session. Conclusion: The evaluated session has no strenuous
metabolic and inflammatory levels.
Keywords: exercise; metabolism;
inflammation; creatine kinase.
Resumo
Introdução: A prática de exercícios físicos tem se
tornado recorrente ao longo da vida na busca pela promoção da saúde. Dentre
inúmeros métodos oferecidos pelo mercado fitness, o Treinamento Corretivo
Postural (TCP) tem por objetivo, por meio de um modelo ginástico, aumentar a
aderência à prática, com reduzido risco de lesões musculoarticulares.
Objetivo: Analisar os efeitos de uma sessão inicial do TCP sobre a lactacidemia, glicemia, frequência cardíaca,
interleucina-6, fator de necrose tumoral- alfa e creatina quinase em indivíduos
saudáveis sem experiência prévia ao método. Métodos: 16 homens ativos, sem
doenças preexistentes (28,5 ± 5,0 anos ;72,16 ± 8,1 kg, 1,75 ±
0,06 m e índice de massa corporal 23,31 ± 2,1) três visitas ao laboratório.
Duas para coleta de dados e uma para realização da sessão do TCP. Resultados:
A sessão apresentou níveis estáveis para as variáveis de glicemia, creatina
quinase, fator de necrose tumoral-alfa e interleucina-6 sanguíneas. Foram
encontradas diferenças significativas para lactacidemia
nos minutos 20’ (4,9 ± 1,5 mmol/L) e 30’ (4,1 ± 1,6 mmol/L) da
sessão. Conclusão: A sessão avaliada não apresenta níveis metabólicos e
inflamatórios extenuantes.
Palavras-chave: exercício físico; metabolismo;
inflamação; creatina quinase.
Introduction
The search for
health promotion and quality of life has been a growing target for human
beings. The change in daily habits, particularly the practice of physical
exercise (PE), has become recurrent. Benefits include improved cardiorespiratory
capacity, muscle hypertrophy, weight loss and others [1].
Among the most
used models, we highlight the high intensity interval training (HIIT),
functional training and resistance training [2]. The characteristics of
performance and the high intensity of training is increasingly distancing these
methods from a sedentary public from regular practice of physical exercise.
Corrective Postural Training (TCP) was created with the goal of increasing
practice adherence, reducing the risks of joint muscle injuries and to offer a
training method, which considers the relationship between the individual and
their environment [3].
The TCP method
aims at biomechanical balance to improve daily activities. Based on the
principles of physical training, its demands occur through dynamic and postural
muscle actions executed in angles and amplitudes which promote better
biochemical and mechanical adaptations [3].
Biochemically,
the instant increase in energy requisition provided by physical exercise results
in physiological adjustments for new metabolic demands [4], which include the
uptake of circulating glucose or glucose obtained via glycolysis of the
muscles/liver to generate energy; lipid mobilization in the face of long-term
activities and lactate production in an intensity-dependent manner [5].
Mechanical
stress can be another response to PE’s demands which results in damage and
inflammation of the muscle tissue [6,7]. Muscle microlesions promote lysis of
muscle tissue causing extravasation of the enzyme creatine kinase (CK) into the
bloodstream, becoming an important muscle injury biomarker [8]. In inflammatory
processes, cytokines such as Interleukin-6 (IL-6) and Tumor Necrosis
Factor-Alpha (TNF-α) alter their levels, conferring an anti-inflammatory
effect in view of the physical activity performed [9].
In view of the
above, the objective of the present study was to analyze the effects of a TCP
session on lactic acid (LAC), glycemia (GLY), heart rate (HR), IL-6, TNF-α
and creatine kinase (CK) in active and healthy men without previous experience
with the method.
Methods
Subjects
Sixteen active
men, without preexisting diseases, took part in the study (28,5 ± 5,0 years
old, 72,16 ± 8,1 kg, 1,75 ± 0,06 m) and body mass index (BMI) 23,31 ± 2,1
kg/m2. Inclusion criteria: male adults (between 20 and 40 years
old), recreationally active (150 weekly minutes) over the last six months.
Exclusion criteria: anabolic steroids users; smokers; cardiovascular disease
history, type 2 diabetes mellitus; systemic arterial hypertension; history of
metabolic disease that advocated the use of drugs capable of affecting the
metabolism of carbohydrates or lipids and/or any change in the lipid profile
and inflammatory markers. Fourteen volunteers were selected (n = 14).
The study was
approved by the Human Research Ethics Committee (CEP) from the Federal
University of São Carlos (opinion No. 65352917.9.0000.5504). All volunteers
signed the Informed Consent Form (ICF).
Experimental design
The selected
subjects took part in three visits to the laboratory at the Department of
Physical Education and Human Motricity (DEFMH) located at the Federal
University of São Carlos (UFSCar). Inside an interval
of approximately 15 days, the visits were characterized by: method
familiarization (F1), acute session of TCP (AS2) and blood collection 24
hours after the session (C3) (Figure 1).
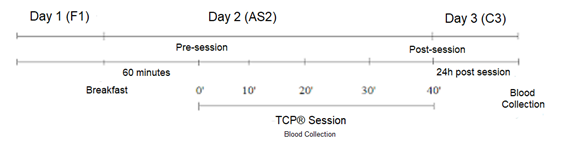
Figure 1 - Experimental
design
Before the
meeting in person, the volunteers answered the anamnesis through an online
questionnaire. The meeting in person (F1) was characterized by signing the
informed consent form (ICF), collecting anthropometric measurements, and
familiarizing the method. After seven days, the second meeting (AS2) (in
fasting and individually), the acute TCP session was performed and blood
collections (TNF-α and IL-6, LAC, GLY, CK) were collected by a qualified
professional. The session took place 60’ after breakfast. 24 hours after the
session, in the third meeting (C3), the last blood collection of CK was carried
out and the volunteers were subsequently dismissed.
Blood variables
Blood
collections were performed on day 2 (SA2) (times: 0’, 10’, 20’ 30’ and 40’ of
the acute session) and 24 hours after the session, on day 3 (C3), as described
in Figure 2.
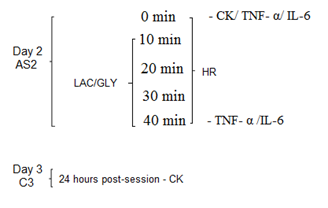
Figure 2 - TCP
Acute Session (AS2), Collection (C3), Heart Rate (HR) / Tumor Necrosis Factor-α
(TNF-α) and Interleukin 6 (IL-6), Creatine Kinase (CK), Lactic acidemia
(LAC) and Glycemia (GLY)
The collected
blood was stored in dry tubes or with EDTA (depending on the analyzed variable)
for approximately 2 hours. Then the tubes were centrifuged at 3000 rpm for
15 minutes at 4ºC, to obtain plasma and/or serum. The inflammatory profile
analyses were carried out in partnership with the Pathology Laboratory of the
Federal University of São Carlos (DMP-UFSCar).
Quantifications were determined by the Immunoenzymatic
Assay (ELISA) method, following the specifications corresponding to the DuoSet ELISA kit. The technique was based on the sandwich
ELISA model: high-affinity microplates were sensitized with monoclonal
anti-cytokine antibodies and remained overnight. Afterwards, they were washed 3
times with 300 µL/well of phosphate buffered saline (PBS) pH 7.2-Tween-20 0.05%
(PBS-Tw), and incubated with the blocking solution containing albumin (PBS pH 7 , 2 + 4% bovine albumin) for 1h, at room temperature (RT).
Then, after another wash cycle, samples were added and
standard curves of recombinant cytokines were made. The plates were kept at RT
for 2 hours and then washed again. Then biotinylated anti-cytokine antibodies
or conjugated with peroxidase enzyme were added and maintained for 1 hour and
30 minutes at RT. After 5 washes with PBS, 100 µl of the developer solution
containing tetramethylbenzidine (TMB) was added. The reaction was blocked with
50 µL of 1 molar sulfuric acid (M) and the reading was carried out in a
spectrophotometer 450 nanomolar (nm). Sample concentrations were calculated
from the titration curve of cytokine standards and final concentrations
expressed in pg/ml.
GLY, LAC and CK
analyses were performed at the Laboratory of Nutrition and Metabolism Applied
to Exercise (LNMAE) at the DEFMH. For GLY and LAC determinations, Roche's
Accu-Chek Active portable equipment, and Roche's Accutrend
Lactate 3012522 mmol/L, respectively, were handled, both calibrated with blood.
For CK analysis, the Roche Reflotron Plus device was
used. For these analyses, blood was collected through a finger puncture with
the Accu-chek Safe-T Pro lancet, from the Roche brand
(subsequently discarded) and applied to the reagent strips. After the period
stipulated for each equipment, the results were extracted.
Heart Rate
HR was recorded
by the Polar monitor between the minute 0’ and 40’ of the session between 5 min
intervals.
TCP Session
The session
consisted of a video class (40 minutes) elaborated with natural, functional,
and coordinating movements simultaneously. Muscle activity was harmoniously
standardized, with acyclic movements and low impact, performed predominantly in
the frontal plane. Some of these movements were: lateral dislocations, flexion,
and extension (knee, elbow, shoulder, and hip) and stationary gait. The musical
frequencies remained between 124 bpm to 132 bpm.
Statistical analysis
Parametric tests
were used for data exhibiting normal distribution (Kolmogorov-Smirnov) and
equality of variance (Levene). The non-parametric
test (Wilcoxon) was used when the data did not present normal distribution
and/or equality of variance (for IL-6 and TNF-α). For pre- and
post-session comparisons of the CK variable, the paired t-test was required.
The parametric test of variance analysis for one factor (one-way ANOVA) was
used to identify possible changes induced by the TCP exercise factor in the LAC
and GLY variables during the session. When statistical difference was detected
by the one-way ANOVA test, Tukey's multiple comparison test (Post-hoc)
was used.
The required
statistical program was SPSS for Windows, version 17.0 (IBM SPSS, Chicago, IL).
The results are presented as mean and standard deviation. The level of
significance was set at p < 0.05. We chose the GraphPad Prism software
(version 6) to make the graphs.
Results
General information regarding the characterization of
the sample is shown in table I.
Table I - Anthropometric
and hemodynamic parameters in physically active adult men
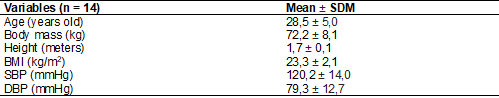
SDM = Standard
Deviation of the Mean; BMI = body mass index; SBP = systolic blood pressure;
DBP = diastolic blood pressure
Regarding
glycemia, the mean over the session was 92.96 ± 5.17 mg/dL (p = 0.57). No
significant differences were found between the collected points (10': 88.5 ±
17.1 mg/dL; 20': 89.7 ± 13.6 mg/dL; 30': 90.6 ± 12.9 mg/dL; powders (40 '):
95.0 ± 10.0 mg/dL) (Figure 3).
The mean value
of the LAC concentration was 3.8 mmol/L changes, compared to the mean values
immediately after the session (2.8 ± 1.1 mmol/L). No significant differences
were found between the 10 'values (3.6 ± 1.0 mmol/L) and immediately after the
session (40') (2.8 ± 1.2 mmol/L) (Figure 3).
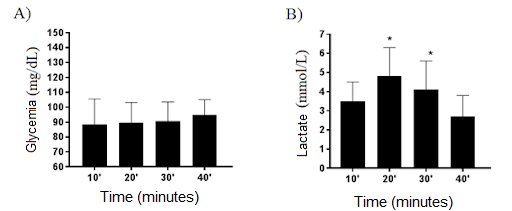
A) Mean blood glucose
values during the TCP class and immediately after the session. One-way ANOVA (p
= 0.57); B) Mean lactacidemia values during the TCP
class and immediately after the session. *20’and 30’ other than time 40’
(immediately after the session). One-way ANOVA, Tukey, F (6.4) (p = 0.00)
Figure 3 - Mean
values of glycemia and lactic acid during the TCP session in physically active
adult men
The plasma levels pre (0') and post-session (40') of
TCP for the cytokines IL-6 and TNF-α did not show significant statistical
changes, as described in the table below.
Table II - Mean
values ± standard deviation of serum levels of IL-6 and TNF-α, in active
adults before and immediately after the TCP session
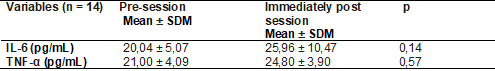
Wilcoxon; SDM =
Standard Deviation of the Mean; IL-6 = interleukin-6; TNF-α = tumor
necrosis factor-alpha
The blood CK
concentration between the pre (0 ') and 24h periods after the TCP session did
not show significant differences as described in the table below.
Table III - Mean
values ± standard deviation of CK blood concentrations in active adults, pre
and 24h after the TCP session

Paired t-test; SDM =
Standard deviation from mean; CK = creatine kinase
Finally, the
mean HR values increased progressively, returning to baseline levels at the end
of the session. Significant values stood out from 10’, remaining until 35’,
when compared to the average values of the initial and final minutes (Figure
4).
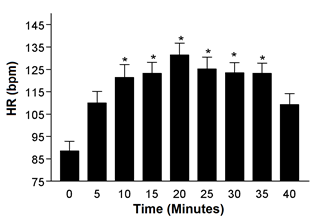
HR = Heart rate; bpm =
beats per minute; * 10', 15', 20', 25', 30', and 35' different from 0', 5',
40', single-factor ANOVA, Tukey, F (6.7) (p = 0.00)
Figure 4 - Mean
heart rate values during the TCP session in physically active adult men
Discussion
The responses
mediated by the acute session of the TCP training model with active and adult
men, were characterized by maintaining stable blood glucose levels, blood CK
concentrations (24h post-session), TNF-α and IL-6 (pre- and post-session).
Significant changes were seen in lactacidemia, with
an increase in times 20 ’and 30’ and for HR between the period 10 ’to 35’,
compared to the rest of the session.
During physical
exercise, in an acute way, the maintenance of energy control for energy generation
occurs mainly through the metabolism of carbohydrates and lipids, coming from
intra and extramuscular substrates. They are: glycogen, blood glucose, fatty acids and triglyceride
reserves of adipose tissue. The intensity and duration of the exercise are the
determinants of the relative contribution of these substrates to oxidative
metabolism. While oxidation of carbohydrates, particularly muscle glycogen,
dominates at higher exercise intensities, fat oxidation stands out at lower
intensities [10].
Blood glucose is
derived from hepatic glycogenolysis, gluconeogenesis and from the intestine
when carbohydrate is ingested. During physical exercise, with the increase in
blood supply, the availability of glucose increases to regulate the use of
energy in the worked muscles. Depending on the intensity performed, glucose is
measured by the concentration available in the bloodstream and/or by
glycogenolysis mainly from the muscle [10]. The entry of glucose into the cell,
allowed primarily by diffusion, is facilitated through GLUT 4, either by the
action of insulin or through adjacent mechanisms of physical exercise
[5,11,12]. Our data showed that glycemic levels during the TCP session remained
unchanged. These results corroborate data from the literature in which they
recorded average blood glucose levels of 95.08 ± 11.55 mg/dL in 40’ in
moderate-intensity aerobic exercise [13]. This stability is possibly due to the
intensity of the session, in which it promoted a balance between the
consumption of muscle glycogen, fatty acids by the muscle in activity with
hepatic glycogenolysis, maintaining the ideal glycemic levels for use as an
energy source by peripheral tissues.
However,
extremely active muscles have accelerated rates of glycolysis, generating
lactate. Depending on the intensity of the exercise, the lactate concentration
increases in the muscle and in the bloodstream in the face of more intense
demands [10]. During the session, between 20' and 30 'times, lactic acid rates
showed a significant increase, compared to the other periods, reaching a
plateau. These results point to a greater intensity of the session, resulting
from the movements performed, involving larger muscle groups. However, the
concentration was not sufficient to cause fatigue, regarding this condition a
physiological response consistent with the effort made. The mean lactic acid
level was 3.57 mmol/L, this result reinforces the idea that the TCP session is
maintained via the oxidative process [14], due to the mean lactate values
remaining within the considered Maximum Stable Lactate Phase (MSLP).
In support of
the findings, data from our study group [14], when measuring energy
expenditure, VO2max and HR in a similar session of TCP modality,
demonstrated that these variables were greater for the same periods measured in
the present study (20 'to 30' of the session), showing that the metabolic
demand was similar in both conditions. The behavior of HR is associated with
the direct response of muscle contraction in view of the need of the active
muscle for oxygen and blood supply for nutrition and removal of metabolites
generated by physical exercise, being proportional to the individual's current
intensity and physical capacity [15]. Concomitantly with the data presented, the
HR means increased progressively, remaining stable between 10 to 35 minutes.
These moments represent the greatest work intensity, however at non-strenuous
levels. As assessed in another study, the same session was of moderate
intensity with mean values of 66% of HRmax [14].
Another way to
assess the intensity of PE is to ascertain its ability to generate muscle
microlesions and inflammation through CK. Biochemically, this enzyme is
involved in the simplest and fastest of the energy systems for resynthesis of
Adenosine Triphosphate (ATP) in generating energy [16,17,18]. Due to its low
molecular weight, CK is the first substance to appear in the circulation
depending on the amount of damage to sarcomeres induced by muscle damage, ischemia,
and inflammation [19]. Although the evaluated TCP session showed a brief
increase in intensity over time, the values obtained for this variable, as
expected, were not able to generate significant muscle damage 24 hours after
the session. Such results can be explained due to the movements performed in
the session being composed of greater recruitment of postural muscles and low
demand for eccentric muscle contraction. According to some authors, static
actions, and especially eccentric ones, as well as the speed of movement are
factors that promote muscle damage [19,20].
Still, it is
observed that the TCP session has a low inflammatory response, since it did not
show changes in the plasma levels of TNF-α and IL-6 immediately after the
session. During the acute session of physical exercise, cytokines are produced
and released to induce an inflammatory response. The cytokine IL-6 when
associated with the immune response is the first signaling molecule synthesized
by macrophages and lymphocytes in conditions of tissue damage or infection. In
the presence of tissue damage, the pro-inflammatory cytokine TNF-α is one
of the main modulators of the acute phase inflammatory response [21,22]. The
kinetics of these cytokines, from muscle contraction immediately after the
session, is dependent on the intensity and duration of the session, muscle
damage, muscle glycogen content and blood glucose [22,23,24,25]. Despite the findings
for the TCP session showing slight increases in the plasma concentration of
IL-6 and TNF-α, the session was not significantly influenced when compared
to the resting values, which demonstrates that the session did not show
strenuous inflammatory levels. These results are similar to
previous studies that have shown small increases or no effect of low or
moderate-intensity acute aerobic exercise on IL-6 and TNF-α [21,26,27,28,29].
Conclusion
Considering the
physiological behavior, through the results obtained from the maintenance of
glycemic levels and mild lactic acid alteration, the evaluated TCP session is
an aerobic session of moderate intensity, with low inflammatory condition and
low muscle damage. Thus, this TCP session can be considered a possible training
session for sedentary individuals, in physical rehabilitation processes, or
even as an active recovery approach for athletes after strenuous physical
exertion. Although there are limited studies in literature about methods and
movements like TCP, further studies are needed on the physiological effects
promoted by the method in other sessions, thus demonstrating its benefits in
the prevention of metabolic diseases.
Potential conflict of
interest
No conflicts of
interest potentially relevant have been reported for this article.
Financing source
Coordination for the
Improvement of Higher Education Personnel (CAPES), through a scholarship, the
researcher Carla Nascimento dos Santos Rodrigues and
the Federal University of São Carlos (UFSCar).
Academic affiliation
This article represents
part of the dissertation of Carla Nascimento dos Santos Rodrigues, supervised
by Professor Dr. Ana Claudia Garcia de Oliveira Duarte from The Federal
University of São Carlos (UFSCar), through the
Graduate Program in Science in Physiological Sciences (PIPGCF)
Author contribution
Conception and design
of the research: Rodrigues CNS, Fabrizzi F; Duarte
ACG. Data obtainment: Rodrigues CNS; Furino
VO; Marine DA; Lima MA. Analysis and
interpretation of data:
Rodrigues CNS; Alves JM, Fabrizzi F; Duarte ACGO. Statistical analysis: Rodrigues
CNS, Marine DA. Financing: no. Writing
of the manuscript:
Rodrigues CNS, Alves JM. Critical review of the manuscript
for important intellectual content: Rodrigues CNS; Furino
VO; Marine DA; Lima MA, Alves JM, Fabrizzi F, Duarte
ACGO.
References
- Ferrari
HG. Domínios de intensidade e sobrecarga metabólica em aulas de Body Pump e Body
Combat. Fitness & Performance Journal 2006;5(6):370-5. doi: 10.3900/fpj.5.6.370.p [Crossref]
- Thompson WR. Worldwide survey of fitness trends for 2017. ACSM's Health & Fitness Journal 2016;20:(6)8-17. doi: 10.1249/fit.0000000000000252 [Crossref]
- Duarte
ACGO. Por que método treinamento corretivo postural
(TCP)® Pressupostos teóricos e princípios práticos básicos. In: V Colóquio de
pesquisa qualitativa em motricidade humana: motricidade, educação e experiência
-CPQMH/II Congresso Internacional de Educação Física Esporte e Lazer-CIEFEL/VI Shoto Workshop [online]; 2012 out 4-6; São Carlos. São
Carlos: Motricidade; 2012. p.141-57. [cited 2021 May
20] Available from:
http://motricidades.org/conference/index.php/cpqmh/5cpqmh/paper/view/2144
- Brum
P, Forjaz C, Tinucci T, Negrão C. Adaptações agudas e
crônicas do exercício físico no sistema cardiovascular. Rev
Paul Educ Fis
2004;(18):21-31.
- Lehninger
A, Nelson D, Cox M. Princípios de Bioquímica. Barcelona:
Omega; 2013.
- Lieber R, Shah S, Fridén J.
Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin
Orthop Relat Res
2002;(403S):90-9. doi: 10.1097/00003086-200210001-00011 [Crossref]
- Petersen A, Pedersen B. The anti-inflammatory effect of exercise. J Appl Physiol 2005;98(4):1154-62. doi: 10.1152/japplphysiol.00164.2004 [Crossref]
- Silva F, Macedo D. Exercício físico, processo inflamatório e adaptação: uma visão geral. Rev Bras Cineantropom Desempenho Hum 2011;13(4). doi: 10.5007/1980-0037.2011v13n4p320 [Crossref]
- Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Kemp BE. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006;55(10):2688-97. doi: 10.2337/db05-1404 [Crossref]
- Hargreaves M, Spriet LL. Skeletal muscle energy metabolism during exercise. Nature Metabolism. Springer Science and Business Media 2020;2(9):817-28. doi: 10.1038/s42255-020-0251-4 [Crossref]
- Pádua M, Pádua T, Pauli J, Souza C, Silva A, Ropelle E, et al. Exercício físico reduz a hiperglicemia de jejum em camundongos diabéticos através da ativação da AMPK. Rev Bras Med Esporte 2009;15(3):179-84. doi: 10.1590/s1517-86922009000300003 [Crossref]
- Irigoyen
M, De Angelis K, Schaan B, Fiorino P, Michelini L. Exercício físico no diabetes melito associado
à hipertensão arterial sistêmica associado à hipertensão arterial
sistêmica. Rev Bras Hipertens 2003;10:109-16.
- Barrile S, Coneglian C, Gimenes C, Conti M, Arca E, Rosa Junior G, et al. Efeito agudo do exercício aeróbio na glicemia em diabéticos 2 sob medicação. Rev Bras Med Esporte 2015;21(5):360-3. doi: 10.1590/1517-869220152105117818 [Crossref]
- Lima
M. Respostas cardiorrespiratórias frente ao método Treinamento Corretivo
Postural. 2018 [Dissertação]. São Carlos: Universidade Federal de São Carlos. [cited 2019 October 15]. Available
from: https://repositorio.ufscar.br/bitstream/handle/ufscar/10777/DISSERTA%C3%87%C3%83O-%20LIMA%2c%20M.A..pdf?sequence=1&isAllowed=y
- Sandoval
AE. Medicina do esporte: princípios e prática. Porto Alegre: ArtMed; 2005.
- Westgard JO, Barry PL, Hunt MR, Groth
T. A multi-rule Shewhart chart for quality control in clinical chemistry. Clin Chem
1981;27(3):493-501.
- Sacher
R, McPherson R. Widman:
Interpretação clínica dos exames laboratoriais. 11ª ed. São
Paulo: Manole; 2002.
- Sayers S, Clarkson P. Short-term immobilization after eccentric exercise. Part II: Creatine Kinase and Myoglobin. Med Sci Sports Exerc 2003;35(5):762-8. doi: 10.1249/01.mss.0000064933.43824.ed [Crossref]
- Koch AJ, Pereira R, Machado M. The creatine kinase
response to resistance exercise. J Musculoskelet
Interact 2014;14(1):68-77.
- Chapman D, Newton M, Sacco P, Nosaka K. Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int J Sports Med 2006;27(8):591-8. doi: 10.1055/s-2005-865920 [Crossref]
- Petersen A, Pedersen B. The anti-inflammatory effect of exercise. J Appl Physio 2005;98(4):1154-62. doi: 10.1152/japplphysiol.00164.2004 [Crossref]
- Pedersen B, Febbraio M. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012;8(8):457-465. doi: 10.1038/nrendo.2012.49 [Crossref]
- Reihmane D, Jurka A, Tretjakovs P, Dela F. Increase in IL-6, TNF-α, and MMP-9, but not sICAM-1, concentrations depend on exercise duration. Eur J Appl Physiol 2012;113(4):851-8. doi: 10.1007/s00421-012-2491-9 [Crossref]
- Rosa Neto J, Lira F, Mello M, Santos R. Importance of
exercise immunology in health promotion. Amino Acids 2010;41(5):1165-72. doi: 10.1007/s00726-010-0786-x [Crossref]
- Febbraio M, Steensberg A, Keller C, Starkie R, Nielsen H, Krustrup P, et al. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol 2003;549(2):607-12. doi: 10.1113/jphysiol.2003.042374 [Crossref]
- Nieman D, Henson D, Smith L, Utter A, Vinci D, Davis J,
et al. Cytokine changes after a marathon race. J Appl Physiol 2001;91:109-14. doi: 10.1152/jappl.2001.91.1.109 [Crossref].
- Neves PRS, Tenório TRS, Muniz M, Valle Neto LM, Botero JP, Oyama LM, et al. Efeitos de diferentes intensidades de exercício sobre a concentração sérica de interleucinas. Rev Bras Educ Fís Esp 2014;28(4):45-52. doi: 10.1590/1807-55092014000400545 [Crossref]
- Magalhães DM, Rocha NP, Vaz LC, Faria MAS, Nunes-Silva A, Rocha NP, et. al. Changes in inflammatory molecules following moderate intensity continuous and high intensity intermittent acute exercises in young healthy men. Annals of Research in Sport and Physical Activity. Coimbra University Press; 2018;(ex2018):160-1. doi: 10.14195/2182-7087_ex2018_54 [Crossref]
- Brown M, McClean CM, Davison GW, Brown JCW, Murphy MH. The acute effects of walking exercise intensity on systemic cytokines and oxidative stress. Eur J Appl Physiol 2018;118(10):2111-20. doi: 10.1007/s00421-018-3930-z [Crossref]