Rev Bras Fisiol Exerc 2021;20(2):212-23
doi: 10.33233/rbfex.v20i2.4410
ORIGINAL ARTICLE
Sarcopenia and overweight in women with systemic lupus
erythematosus
Sarcopenia
e excesso de peso em mulheres portadoras de lúpus eritematoso sistêmico
João
Ronaldo Silva Monteiro, Maria Cecília Costa Moreira Cardoso, Alane Cabral Menezes de Oliveira, Juliana Célia de Farias
Santos
Universidade
Federal de Alagoas, Maceió, AL, Brazil
Received:
October 13, 2020; Accepted:
March 8, 2021.
Correspondence: Juliana Célia de Farias Santos, Avenida Lourival de Melo
Mota, Universidade Federal de Alagoas/ Faculdade de Nutrição, Campus AC Simões,
BR 104 Norte, Km 96,7 Tabuleiro dos Martins 57072-970 Maceió AL
João Ronaldo Silva Monteiro: joaomonteironutri@outlook.com
Maria Cecília Costa Moreira Cardoso:
mariaceciliac@outlook.com
Alane Cabral Menezes de
Oliveira: alanecabral@gmail.com
Juliana Célia de Farias Santos: jcfsnut@hotmail.com
Abstract
Objective: To investigate the prevalence of
sarcopenia according to the categories of body mass index (BMI) in women with
systemic lupus erythematosus (SLE) assisted by a teaching hospital in Maceió, Alagoas, Brazil. Methods: Cross-sectional
analysis with patients selected by convenience, which included socioeconomic,
demographic, clinical, anthropometric and sarcopenia data. The anthropometric
evaluation included BMI, body circumferences, skin folds, bioimpedance analysis
and fat percentage. Sarcopenia was assessed according to the diagnostic
criteria proposed by the European Working Group on Sarcopenia in the
Elderly-EWGSOP (2019), which includes a SARC-F screening protocol, muscle
strength, muscle mass and physical performance. Pearson's chi-square test was
distributed, adopting a significance level of p < 0.05 and a 95% confidence
interval. Results: 62.8% of the women presented overweight, followed by
32.5% with normal weight and 4.6% with malnutrition. Sarcopenia was not
detected in our sample. However, the SARC-F screening identified 17.5% possible
cases of sarcopenia, while 21.4% of the patients had probable sarcopenia
according to the criteria of low handgrip strength (HGS). Still, a portion of
the sample showed reduction in physical performance, with no statistical
differences according to the BMI categories. Also, 66.6% of women with probable
sarcopenia and all those screened by SARC-F for sarcopenia, presented
overweight. Conclusion: The reduced muscle strength, performance and the
high weight are an alert do decrease in muscle functionality, making clear the
need for early care of this population as well as adaptations of the sarcopenia
assessment instrument for SLE.
Keywords: autoimmune disease; overweight;
obesity; skeletal muscle; adipose tissue.
Resumo
Objetivo: Investigar a prevalência de sarcopenia
de acordo com as categorias de índice de massa corporal (IMC) em mulheres com
lúpus eritematoso sistêmico assistidas por um hospital de ensino de Maceió,
Alagoas. Métodos: Análise transversal com pacientes selecionadas por
conveniência, que incluiu dados socioeconômicos, demográficos, clínicos,
antropométricos e de sarcopenia. A avaliação antropométrica incluiu IMC,
circunferências corpóreas, pregas cutâneas, análise de bioimpedância e
percentual de gordura. A sarcopenia foi avaliada segundo os critérios
diagnósticos propostos pelo European Working Group on
Sarcopenia in Older People-EWGSOP
(2019), que inclui um protocolo de triagem SARC-F, a força muscular, a
quantidade muscular e o desempenho físico. Foi aplicado o teste de qui-quadrado de Pearson, adotando nível de significância de
p < 0,05 e intervalo de confiança de 95%. Resultados: 62,8% das
mulheres apresentaram excesso de peso, seguidas de 32,5% com eutrofia e 4,6%
com desnutrição. Não foi detectada sarcopenia em nossa amostra. Contudo a
triagem SARC-F apontou 17,5% possíveis casos de sarcopenia, enquanto 21,4% das
pacientes tiveram provável sarcopenia de acordo com o critério de baixa força
de preensão manual (FPM). Ainda, uma parcela da amostra apresentou redução de
desempenho físico, sem diferenças estatísticas de acordo com as categorias de
IMC. Ainda, 66,6% das mulheres com provável sarcopenia e todas aquelas triadas
pelo SARC-F para sarcopenia, apresentaram excesso de peso. Conclusão: O
desempenho, a força muscular diminuída e o elevado excesso de peso são alertas
para a diminuição de funcionalidade muscular, ficando claro a necessidade do
cuidado precoce desta população, bem como adaptações do instrumento avaliativo
de sarcopenia para o LES.
Palavras-chave: doença autoimune; sobrepeso;
obesidade; músculo esquelético; tecido adiposo.
Introduction
Systemic lupus
erythematosus (SLE) is a chronic and autoimmune inflammatory disease,
characterized by the deposition of immune complexes in several organs, leading
to tissue damage [1]. Although genetic, environmental, hormonal, and
pharmacological factors may play an important role in the development and
clinical course of the disease, its etiopathogenesis is still unclear [1,2].
During periods
of exacerbation, in view of the multisystemic nature of SLE, the disease may
present itself in different ways. Skin and joint changes, pleuritis,
pericarditis, nephritis, neuropsychiatric and hematological changes are
commonly identified and may vary according to the predisposition of each
patient affected by the disease [1,3].
In addition to
these, skeletal muscle changes can affect the clinical course of SLE and the
quality of life of these patients [4]. Among these changes, sarcopenia has been
highlighted, defined as a progressive and generalized disorder of skeletal
musculature characterized by low muscle quantity and/or quality, accompanied by
low muscle strength and low physical performance, the latter being used as a
measure of the severity of this syndrome [5]. Moreover, this disorder is common
with advancing age, but it can also be a result of chronic non-communicable
diseases (CNCDs) contributing to the development of complications and adverse
outcomes [5,6,7]. In addition to sarcopenia, other changes in body composition
can be detected and include abnormalities in nutritional status. An increase in
the prevalence of overweight / obesity and a proportion, albeit lower, of
malnutrition have been identified [8].
In view of the
compromised quality of life resulting from SLE, patients who manifest
musculoskeletal and body composition changes are dependent on the performance
of routine activities and greater physical inactivity, greater perception of
pain in muscles and joints, greater neurocognitive impairment, increased risk
of fracture, increased risk of cardiovascular complications, metabolic syndrome,
and ovarian failure, further compromising quality of life [9,10,11,12,13,14,15].
Data available
in the literature on the factors that corroborate the development and worsening
of disorders in body composition and conservation of muscle mass point out that
among such factors are the use of glucocorticoids, the metabolic changes
inherent to the disease and the food profile [16,17,18].
In clinical
practice, changes in body composition can occur independently of changes in
body mass index (BMI), which suggests a difficulty in diagnosing sarcopenia
influenced by adequate nutritional status or being overweight [12,16]. Knowing
this, the present study aimed to describe the prevalence of sarcopenia
according to the categories of BMI in women with SLE assisted by a teaching
hospital in Maceió, Alagoas.
Methods
This is a
cross-sectional study that is part of a larger project entitled “Sarcopenia in
Systemic Lupus Erythematosus”, approved by the Ethics and Research Committee of
the Universidade Federal de Alagoas
(Opinion No. 3.138.940/CAAE 89436418.1.0000.5013). The collections took place
between August 2018 and September 2019 at the Integrated Center for Nephrology
at the Professor Alberto Antunes University Hospital, according to all ethical
criteria. The sample consisted of 43 female patients selected for convenience,
including those over the age of 18, with a previously established medical
diagnosis of SLE according to the recommendations of the American College of
Rheumatology [3]. Elderly, pregnant patients, with cancer and HIV positive
serology, hepatitis B and C were excluded from the sample.
Socioeconomic
and demographic variables included age, place of birth and origin, marital
status (with or without a stable union), race (black and non-black), functional
illiteracy over time of schooling (≤ 4 years) [19], employment
relationship, family income (≤ 1 minimum wage/month and> 1 minimum
wage/month), per capita income (≤ ½ minimum wage/month and > ½ minimum
wage/month) and access to piped water. In addition, the socioeconomic
characterization protocol of the Brazilian Association of Research Companies
(ABEP) [20] was applied, through which the stratification of the population
studied was made into classes.
Regarding the
clinical variables, the presence of systemic arterial hypertension (SAH),
diabetes mellitus (DM), other autoimmune diseases, lifestyle (smoking, alcoholism and physical activity), use of medications
(immunosuppressants, antimalarials and corticosteroids), family background of
LES, urinary anamnesis (foam in the urine and hematuria), presence of edema
were investigated.
The
anthropometric evaluation included the calculation of BMI, waist circumference
(WC) and percentage of fat. BMI, calculated from weight divided by height
squared (kg/m²), was classified as malnutrition (< 18.5 kg/m²), eutrophy (≥ 18.5 to < 25 kg/m²), overweight (≥
25 to < 30 kg/m²) and obesity (≥ 30 kg/m²) [21]. WC was adopted as a
measure of cardiovascular risk (CVR) [22]. The fat percentage was calculated
from the sum of 4 skin folds and classified, according to sex and age group,
having been dichotomized as above average or on average or below average
[23,24]. Overweight and obesity were grouped into a single category of
overweight when the BMI classification was made.
In the present
study, the assessment of sarcopenia followed the algorithm proposed by the
European Working Group on Sarcopenia in Older People (EWGSOP2, 2019) [5]. The
consensus, recently revised, includes a screening protocol and three criteria
for the definition and diagnosis of sarcopenia, namely: low muscle strength,
low muscle strength and low physical performance.
Screening for
sarcopenia was done with SARC-F. This is a screening protocol composed of 5
components (strength, assistance in walking, getting up from a chair, climbing
stairs and falls) that has a very high specificity that mainly predicts severe
cases of sarcopenia. Patients who scored ≤ 4 were at risk for sarcopenia.
The probable
sarcopenia was detected from low muscle strength (< 16 kg/force), assessed
by handgrip strength (HGS). This, in turn, was measured with a manual hydraulic
dynamometer with a scale from 0 to 90 kg and a resolution of 2 kg, for which
the highest value of three measurements, in kg/force, of the dominant hand was
adopted. The measurements were performed with the arm positioned following the
shoulder line, forming a 90° angle between the arm and forearm. During the
procedure, the participants were verbally encouraged to squeeze as hard as
possible.
To confirm the
diagnosis of sarcopenia, the low muscle quantity (< 5.5 kg/m²) was measured
with a portable tetrapolar bioelectrical impedance device (BIA) operating in 50
kHz mono-frequency. To perform the test, the patients were placed in the supine
position and should meet according to the conditions required by the device.
The arithmetic means of three measurements were adopted, and the resistance
values then obtained were applied to estimate the appendicular skeletal muscle
mass (ASMM) using the Janssen formula [25]. The MMEA value obtained was
adjusted for the size of the body, dividing it by height, in meters squared.
Physical
performance was assessed by 3 different methods. In the Timed-UP and GO (TUG)
the evaluated patients got up from a chair and walked to a marked point 3
meters away and returned to the chair as the time spent between getting up and
sitting in the chair at the end of the test was timed. As for the gait speed
method, expressed in meters per second, the patients traveled at the usual
speed at 4 meters as time was measured. In the Short Battery of Physical
Performance (SPPB), which includes walking speed, patients were asked to
perform a balance test (in which they had to remain balanced for a minimum of
10 seconds) and a chair position test (in which the act of sitting and getting
up from the chair was requested in 5 repetitions, with a score being assigned
according to the time required by each patient to complete this test or even
its interruption through some limitation). To diagnosing severe sarcopenia, a
low gait speed (≤ 0.8 m/s) was used.
The collected
data were tabulated and analyzed using the SPSS version 20.0 statistical
package, adopting a 95% confidence interval. Pearson's chi-square test was performed,
for which a significance level of p < 0.05 was adopted, and analysis of
variance (ANOVA). Still, the results were expressed by means of descriptive
statistics (frequencies, means and standard deviation (SD)).
Results
The
socioeconomic, lifestyle, anthropometric, clinical characterization are shown
in more detail in Table I. 43 patients with SLE with a mean age of 34.67 ± 8.67
years were included. The highest proportion of patients who declared themselves
black (88.4%), the highest proportion of participants who fell in class C
(51.2%), followed by those in class D (39.5%) is noteworthy. When asked about
their lifestyle, the proportion of patients who denied their current practice
or history of smoking, alcoholism and regular physical activity was higher. Out
of the clinical conditions investigated, lupus nephritis was the most prevalent
(53.6%). On physical examination, the occurrence of edema (35.7%) was
identified, something already expected in our patients due to drug therapy
(100% of patients using autoimmune medication and almost 70% using
corticosteroids). As for anthropometric data, a higher prevalence of CVR was
identified (76.7%), a higher proportion of high fat percentage (86.6%) and a
higher prevalence of overweight in terms of nutritional status (62.8%).
Table I - Socioeconomic, clinical,
lifestyle and anthropometric characterization of women with systemic lupus
erythematosus assisted by a teaching hospital in Maceió/AL,
2018-2019
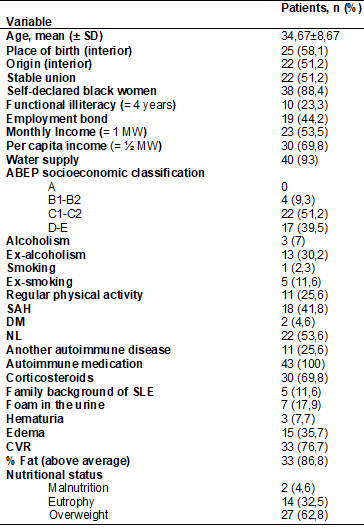
N = absolute value; SD
= standard deviation; SM = minimum wage; ABEP = Brazilian Association of
Companies and Research; SAH = systemic arterial hypertension; DM = diabetes
mellitus; NL = lupus nephritis; SLE = systemic lupus erythematosus; CVR =
cardiovascular risk
As for the
assessment of sarcopenia, the SARC-F screening data revealed 17.5% of possible
cases of sarcopenia. When applied the criteria of low muscular strength,
detected from the low HGS, the probable sarcopenia was pointed out in 21.4% of
the patients. Sarcopenia, which was formally confirmed by the association of
the previous parameter with low ASMM, was not detected in any of the patients.
An important observation was that all possible cases of sarcopenia identified
by the SARC-F instrument were from the overweight / obesity group. In addition,
66.6% of patients with probable sarcopenia also belonged to this group (Table
II). Statistical difference, between groups by BMI, was only observed for MMEA
(p < 0.012) (Table III), with the obese group presenting a higher ASMM than
the eutrophic group (p <0.046, Tukey), data not shown.
In addition to
low muscle strength, impaired physical performance was also detected, with a
higher proportion in the overweight / obesity group as observed in the low TUG
score (66.6%), low SPPB score (77.7%) and low speed gait (66.6%) (Table III).
Table II - Diagnosis of sarcopenia by
BMI category, according to the EWGSOP (2019), in patients with systemic lupus
erythematosus assisted by a teaching hospital in Maceió/AL,
2018-2019
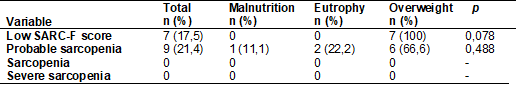
Pearson's chi-square
test (Statistical significance = p <0.05); (-): statistics not computed due
to the constant occurrence of the variables; n = absolute value; SARC-F =
simple five-component questionnaire
Table III - Parameters for assessing
Sarcopenia according to the EWGSOP (2019), by BMI category, in patients with
systemic lupus erythematosus assisted by a teaching hospital in Maceió/AL, 2018-2019
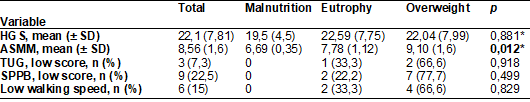
Pearson's chi-square
test (Statistical significance = p < 0.05); n = absolute value; SD =
Standard deviation; *Anova (Statistical significance
= p < 0.05); HGS = handgrip strength (expressed in kilograms / force); ASMM
= appendicular skeletal muscle mass (expressed in kg/height2; TUG = Timed-Up
and Go; SPPB = Short Physical Performance Battery
Discussion
The inflammatory
nature of SLE and corticosteroid therapy can affect body composition,
compromising the conservation of skeletal muscle mass, in addition to favoring
weight gain in the form of body fat. In the context of SLE, these changes in
body composition can occur independently of BMI, making it difficult to
diagnose possible sarcopenia [16]. Despite the literature discussing the
changes in body composition caused by SLE and its treatment, in our study there
was no sarcopenia. Unlike the work carried out in Baghdad, which found a 35%
prevalence of sarcopenia in women with SLE, although it did not differ from the
controls, it was statistically associated with the percentage of fat mass [26].
Among the
reasons that can help in the interpretation of the finding of our study is the
fact that, in the vast majority, the patients included in the analyzes were of
childbearing age, in addition to the fact that no elderly patient was included.
The reduction of sex hormones (testosterone, estrogen and
dehydroepiandrosterone-DHEA), common with advancing age, especially in the
post-menopausal period, may have implications for the conservation of skeletal
muscle mass [27]. However, despite the conservation of skeletal muscle mass, we
identified impaired muscle functionality, pointing to a possible limitation of
the evaluation method for sarcopenia in our patients.
The
inconsistencies that exist between consensus, which establish not only
different assessment methods, but also different cutoff points for the
diagnosis and classification of sarcopenia [28], hinder our assessment. It is
worth mentioning that there are no methods for diagnosing sarcopenia adapted by
pathologies. According to the EWGSOP update, in 2019, it is suggested that, in
our sample, muscle strength may be a more important parameter than the amount
of skeletal muscle capable of pointing out muscle damage. Physical strength is
a more reliable parameter in the assessment of function and reduces more
rapidly when compared to the simultaneous loss of muscle mass, covering the
impact of sarcopenia in this young and female population.
The level of
disease activity can also influence body composition. The metabolic changes
inherent in SLE involve the elevation of pro-inflammatory cytokines (tumor
necrosis factor-α (TNF-α), leukins-6 and -1) that are responsible for
the reduction of muscle protein synthesis and anabolic factors such as growth
hormone (GH) and insulin-like growth factor 1 (IGF-1), in addition to causing
anorectic effects. Although it was not a variable investigated in the present
study, none of the patients reported exacerbation of SLE symptoms, which may
also explain the non-occurrence of sarcopenia in the sample [10,18]. Another
important point is that the evaluated group has continuous treatment by a
specialized group within the hospital, which may also have interfered with the
results. Despite all the limitations of the Unified Health System (SUS), the
hospital is a reference center in the state of Alagoas.
According to
EWGSOP2 (2019), 21.4% had low muscle strength, that is, probable sarcopenia,
which already refers patients to cause assessment and interventions, in
addition to decreased physical performance. These data suggest the occurrence
of other possible syndromes that may affect muscle function, such as dinapenia, frailty syndrome, among others, which, although
identified as geriatric syndromes, deserve to be studied and understood in the
context of SLE [5,29]. Still, the reduction in strength and physical
performance was more common in overweight patients. Excess adiposity can have
an impact on muscle quality and, thus, on physical function (strength and
performance). The deposition of fat between muscle fibers leads to
mitochondrial dysfunction to increase lipid peroxidation and, therefore, metabolic
intermediates and reactive oxygen species (ROS), causing insulin resistance
(IR), increased oxidative stress and lipotoxicity in
the myocyte leading to dysfunction or apoptosis of these cells [30,31,32].
In our sample,
excess weight was more frequent (62.8%). Obesity has been observed in patients
with lupus and there are numerous causes attributed to this outcome, among
which the reduction of the basal metabolic rate, the reduction in the levels of
physical activity, the inflammatory nature of the disease, the therapy with
corticosteroids can be pointed out [4,12,16,18]. Like this work, a study
developed in Minas Gerais that evaluated the nutritional status and the level
of physical activity in patients with SLE, found a higher prevalence of overweight
(35.9%) and obesity (28.3%) [18]. Another study that characterized SLE patients
residing in Malta, identified a prevalence of 31.5% of overweight and 29.3% of
obesity [8].
In the United
States, a study carried out with black women evaluated obesity as a risk factor
for SLE and found a prevalence of 31.6% and 30.2% of overweight and obesity,
respectively [33]. Still in this study, overweight in adolescence was
associated with the development of SLE in adulthood, however the authors draw
attention to the biological mechanisms and exposure windows between obesity and
SLE in black women. It is interesting to mention that studies have shown a
higher prevalence of SLE and risk of mortality in black women when compared to
white women, and that in addition to obesity, psychological stress during
childhood may also be associated with the development of the disease [34,35].
Obesity may be
associated with SLE severity and activity and an increased risk of developing
the disease, although little is known about the pathophysiological mechanisms
involved. The excess of adiposity could increase the levels of serum
inflammatory markers and in this context the adipokines could play an important
role. A leptin has been associated with a T helper 1 (Th1) lymphocyte response
culminating without an increase in pro-inflammatory cytokines as a TNF-α,
increased survival of autoreactive T lymphocytes through Bcl
expression - 2, reduction of regulatory T cells and higher SLE disease activity
index scores (SLEDAI). Resistin, also elevated in
autoimmune diseases, especially in rheumatoid arthritis, is related to greater
inflammation, higher BMI, in addition to being correlated with renal
dysfunction markers in SLE [10,12,33,36].
In view of what
has been discussed so far, the impact of SLE on quality of life is evident, not
to mention the costs, individual and collective, for health that include access
to medicines, exams and consultations that can compromise the necessary
uninterrupted support [9,37]. In this context, individualized dietary
management and nutritional guidelines promote improvements in eating habits
that can help control the disease [38]. Modulations in the diet may include
caloric deficit, adequate protein supply, reduction of total fats contemplating
the supply of w-3
polyunsaturated fatty acids (PUFA w-3),
supplementation of vitamin A, vitamin D, complex B vitamins, especially folate,
B6 and B12, vitamin C, adequacy in the supply of vitamin E, a diet rich in
selenium, adequate in calcium and supplementation when necessary, adjusted in
sodium and limited in iron. The benefits of such strategies include the control
of disease activity, improvement of immune function and control of the
inflammatory condition of SLE marked by the reduction of circulating
autoantibodies and their deposition in tissues, in addition to the reduction of
pro-inflammatory cytokines, suppression of macrophage activity, reduction of
oxidative stress markers [17,39]. Individuals with SLE, especially those with
cardiovascular risk factors such as obesity, SAH, DM and metabolic syndrome,
can benefit from such dietary modulations [40].
Aware of the
limitations of the present study, we can point out those related to the sample
itself, which, due to the use of antihypertensives, water retention resulting
from corticotherapy, can influence the results
obtained in BIA, a method of greater financial accessibility when compared to
DXA. The presence of other chronic conditions hindered our understanding of the
impact of SLE, as well as being overweight on the conservation of lean mass, or
more specifically in this case, the muscle function that was compromised in the
sample, since sarcopenia and other chronic conditions share of many mechanisms
involving a chronic inflammatory condition. Finally, the authors point out the
dependence on a larger research group, which limited data collection with a
larger number of participants.
Conclusion
In this study,
sarcopenia was not identified, however, low muscle strength and reduced
physical performance were identified. A higher prevalence of overweight was
also identified. Such data draw attention to the need for further studies on
the relationship of body composition and the deterioration of muscle
functionality. For this, it is suggested that the nutritional assessment and
diagnostic tools for sarcopenia are adapted considering the particularities of
patients with SLE, such as water retention and periods of remission and
exacerbation of the disease, which will allow the identification of excess
adiposity, as well as the deterioration of muscle mass and/or function, in order to assist in early and individualized therapeutic
interventions.
Acknowledgments
We thank the Integrated
Center for Nephrology and collaborators for welcoming the research group and
patients for their acceptance and contribution to the study.
Financing
No funding source.
Conflict of interests
The authors declare no
conflict of interest.
Authors´s contributions
Conception and design
of the research: Monteiro JRS, Cardoso MCCM, Santos JCF; Data collection:
Monteiro JRS, Cardoso MCCM, Santos JCF; Analysis and interpretation of data:
Monteiro JRS, Cardoso MCCM, Santos JCF; Statistical analysis: Oliveira ACM;
Writing of the manuscript: Monteiro JRS; Critical revision of the manuscript
for important intellectual content: Cardoso MCCM, Santos JCF, Oliveira ACM.
Referências
- Klumb
EM, Silva CAA, Lanna CCD, Sato EI, Borba EF, Brenol JCT, et al. Consenso da Sociedade Brasileira de
Reumatologia para o diagnóstico, manejo e tratamento da nefrite lúpica. Rev Bras
Reumatol 2015;55(1):1-21. doi: 10.1016/j.rbr.2014.09.008 [Crossref]
- Enríquez-Mejía
MG. Fisiopatología del lupus eritematoso sistémico. Rev Med Inv
[Internet] 2013;1(1):8-16. [cited 2021 May 12]. Available from:
https://www.elsevier.es/es-revista-revista-medicina-e-investigacion-353-pdf-X2214310613653982
- Borba EF, Latorre LC, Carlos J, Brenol T, Kayser C, Antonio N, et al. Consenso de lúpus eritematoso sistêmico consensus of systemic lupus erythematosus. Rev Bras Reumatol 2008;55(1):196-207. doi: 10.1590/S0482-50042008000400002 [Crossref]
- Shamekhi
Z, Habibagahi Z, Ekramzadeh
M, Ghadiri A, Namjoyan F, Malehi AS, et al. Body composition and basal
metabolic rate in systemic lupus erythematosus patients. Egypt Rheumatol 2017;39(2):99-102. doi: 10.1016/j.ejr.2016.10.004 [Crossref]
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48(4):16-31. doi: 10.1093/ageing/afz046 [Crossref]
- Costa
TMRL, Costa FM, Jonasson TH, Moreira CA, Boguszewski CL, Borba VZC. Body composition
and sarcopenia in patients with chronic obstructive pulmonary disease. Endocrine
2018;60(1):95-102. doi: 10.1007/s12020-018-1533-4 [Crossref]
- Adams DW, Gurwara S, Silver
HJ, Horst SN, Beaulieu DB, Schwartz DA, et al. Sarcopenia is common in
overweight patients with inflammatory bowel disease and may predict need for
surgery. Inflamm Bowel Dis 2017;23(7):1182-6. doi:
10.1097/MIB.0000000000001128 [Crossref]
- Magro R,
Borg AA. Characterisation of patients with systemic
lupus erythematosus in Malta: a population based cross-sectional cohort study.
BioMed Res Int 2018:2385386. doi: 10.1155/2018/2385386 [Crossref]
- Dixon J, Cardwell FS, Clarke AE, Elliott SJ. Choices
are inevitable: A qualitative exploration of the lifecosts
of systemic lupus erythematosus. Chronic Illn 2020.
doi: 10.1177/1742395320910490 [Crossref]
- Teh P, Zakhary B, Sandhu VK. The impact of obesity on SLE disease activity: findings from the Southern California Lupus Registry (SCOLR). Clin Rheumatol 2019;38(2):597-600. doi: 10.1007/s10067-018-4336-3 [Crossref]
- Katz P, Julian L, Tonner MC, Yazdany
J, Trupin L, Yelin E, et
al. Physical activity, obesity, and cognitive impairment among women with
systemic lupus erythematosus. Arthritis Care Res 2012;64(4):502-10. doi:
10.1002/acr.21587 [Crossref]
- Santos FMM, Borges MC, Correia MITD, Telles RW, Lanna CCD. Assessment
of nutritional status and physical activity in systemic lupus erythematosus
patients. Rev Bras Reumatol 2010;50(6):631-8. doi: 10.1590/S0482-50042010000600004 [Crossref]
- Santos
MJ, Vinagre F, Silva JC, Gil V, Fonseca JE. Body composition
phenotypes in systemic lupus erythematosus and rheumatoid arthritis: a
comparative study of Caucasian female patients. Clin Exp Rheumatol
[Internet] 2011;29:470-6. [cited 2021 May 12]. Available from: https://pubmed.ncbi.nlm.nih.gov/21640047/
- Dey M, Bukhari M. Predictors of fracture risk in
patients with systemic lupus erythematosus. Lupus 2019;27(9):1547-51. doi:
10.1177/0961203318768886 [Crossref]
- Patterson SL, Schmajuk G,
Jafri K, Yazdany J, Katz P. Obesity is independently
associated with worse patient-reported outcomes in women with systemic lupus
erythematosus. Arthritis Care Res 2019;71(1):126-33. doi: 10.1002/acr.23576 [Crossref]
- Mok CC, To CH, Ma KM. Changes in body composition after glucocorticoid therapy in patients with systemic lupus erythematosus. Lupus 2008;17(11):1018-22. doi: 10.1177/0961203308093552 [Crossref]
- Klack
K, Bonfa E, Neto EFB. Dieta e aspectos nutricionais no lúpus eritematoso sistêmico. Rev
Bras Reumatol
2012;52(3):395-408. doi: 10.1590/S0482-50042012000300009 [Crossref]
- Cerpa-Cruz
S, Castañeda-Ureña M, Martinez-Bonilla
G, González-Diaz V, Ruiz-González JF, Pérez-Romero MA, et al. Sarcopenia
in patients with autoimmune diseases. Rev Med MD [Internet] 2016;7(3):136-42.
[cited 2020 sept 10]. Available from:
https://www.medigraphic.com/cgi-bin/new/resumenI.cgi?IDARTICULO=65559
- Instituto
Paulo Montenegro. 5º Indicador Nacional Analfabetismo Funcional. Um diagnóstico
para a inclusão social pela educação. [Internet] São Paulo, 2005. [cited 2020 sept 10]. Available from:
https://ipm.org.br/relatorios
- Associação
Brasileira de Empresas de Pesquisa. Critério de Classificação Econômica Brasil.
[Internet] 2018. p. 1-6. [cited 2020 sept 10]. Available from:
http://www.abep.org/criterio-brasil
- World Health Organization. Physical status: the use
and interpretation of anthropometry, report of a WHO expert committee.
[Internet]. Geneva: WHO; 1995. [cited 2020 sept 10]. Available from:
https://apps.who.int/iris/handle/10665/37003
- World Health Organization. Waist circumference and
waist-hip ratio: Report of a WHO Expert Consultation, Geneva, 8-11 December
2008. [Internet] Geneva: WHO; 2011. [cited 2021 May 12]. https://www.who.int/publications/i/item/9789241501491
- Durnin JV,
Womersley J. Body fat assessed from total body density and its estimation from
skinfold thickness: measurements on 481 men and women aged from 16 to 72 years.
Brit J Nutr
1974;32(1):77-97. doi: 10.1079/BJN19740060 [Crossref]
- Pollock
ML, Wilmore JH, Fox III S. Exercício na saúde e na
doença: Avaliação e prescrição para prevenção e avaliação. Rio
de Janeiro: Medsi; 1993.
- Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioeletrical impedance analysi. J Appl Physiol 2000;89:465-71. doi: 10.1152/jappl.2000.89.2.465 [Crossref]
- Gorial FI, Mahmood ZA, Al Obaidi S. Body composition in Iraqi Women with systemic lupus erythematosus. Glob J Health Sci 2019;11(1):63-70. doi: 10.5539/gjhs.v11n1p63 [Crossref]
- Maggio M, Lauretani F, Ceda GP. Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr Metab Care 2013;16(1):3-13. doi: 10.1097/MCO.0b013e32835b6044 [Crossref]
- Gupta S, Dhillon RJ, Hasni S. Sarcopenia: a rheumatic disease? Rheum Dis Clin of North Am 2018;44(3):393. doi: 10.1016/j.rdc.2018.03.001 [Crossref]
- Tournadre A,
Vial G, Capel F, Soubrier M, Boirie
Y. Sarcopenia. Joint Bone Spine 2019;86(3):309-14. doi:
10.1016/j.jbspin.2018.08.001 [Crossref]
- Carter CS, Justice JN, Thompson L. Lipotoxicity,
aging, and muscle contractility: does fiber type matter? GeroScience
2019;41(3):297-308. doi: 10.1007/s11357-019-00077-z [Crossref]
- Polyzos SA, Margioris AN. Sarcopenic obesity. Hormones (Athenes) 2018;17(3):321-31. doi: 10.1007/s42000-018-0049-x [Crossref]
- Barazzoni R, Bischoff S, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: Time to meet the challenge. Obes Facts 2018;11(4):294-305. doi: 10.1159/000490361 [Crossref]
- Cozier YC, Barbhaiya M,
Castro-Webb N, Conte C, Tedeschi S, Leatherwood C, et al. A prospective study
of obesity and risk of systemic lupus erythematosus (SLE) among black women.
Semin Arthritis Rheum 2018;48(6):1030-4. doi: 10.1016/j.semarthrit.2018.10.004 [Crossref]
- Gómez-Puerta JA, Barbhaiya M, Guan H, Feldman C, Alarcón GL, Costenbader
KH. Racial/ethnic variation in all-cause mortality among United States
Medicaid recipients with systemic lupus erythematosus: a Hispanic and Asian
paradox. Arthritis Rheumatol 2015;67(3):752-60. doi:
10.1002/art.38981 [Crossref]
- Cozier YC, Barbhaiya M, Castro-Webb N, Conte C, Tedeschi S, Leatherwood C, et al. Association of child abuse with systemic lupus erythematosus in Black women during adulthood. Arthritis Care Res 2020. doi: 10.1002/acr.24188 [Crossref]
- Urrego
T, Vásquez GM, Gómez-Puerta JA. Relationship
between obesity, adipokines and systemic lupus erythematosus. Rev Fac Cienc Med (Cordoba, Argentina)
[Internet] 2016;73(1):32-9. [cited 2021 May 12]. Available from: https://pubmed.ncbi.nlm.nih.gov/27419894/
- Santos
LMO, Vilar MJ, Maia EMC. Mulheres com lúpus eritematoso sistêmico, sintomas
depressivos e apoio social. Psic Saúde Doenças
2017;18(1):39-54. doi: 10.15309/17psd180104 [Crossref]
- Abad
TO, Sarni RO, Silva SG, Machado D, I Suano-Souza F, Len CA, et al. Nutritional intervention in
patients with juvenile systemic lupus erythematosus: protective effect against
the increase in fat mass. Rheumatol
Int 2018;38(6):985-92. doi: 10.1007/s00296-018-4031-3 [Crossref]
- Borges
MC, Santos FMM, Teles RW, Andrade MVM, Correia MITD, Lanna
CCD. Ácidos graxos ômega-3, estado inflamatório e marcadores bioquímicos de
pacientes com lúpus eritematoso sistêmico: estudo piloto. Rev
Bras Reumatol
2017;57(6):526-34. doi: 10.1016/j.rbre.2016.09.014 [Crossref]
- Robinson GA, Mcdonnell T, Wincup C, Martin-Gutierrez L, Wilton J, Kalea AZ, et al. Diet and lupus: what do the patients think? Lupus 2019;28(6):755-63. doi: 10.1177/0961203319845473 [Crossref]