Rev Bras Fisiol exerc 2021;20(3):293-303
doi: 10.33233/rbfex.v20i3.4550
ORIGINAL ARTICLE
Evaluation of serum arginase in overweight women under
the acute effect of physical exercise - exploratory study
Avaliação
da arginase sérica em mulheres com excesso de peso
sob o efeito agudo do exercício físico – estudo exploratório
Hiole
Gonçalves Nolasco1, Djeyne Silveira
Wagmacker2, Jacqueline de Jesus Silva3, Amâncio José de
Souza1, Ana Marice Teixeira Ladeia1
1Escola Bahiana
de Medicina e Saúde Pública, Salvador, BA, Brazil
2Faculdade Adventista da Bahia, Capoeiruçu, BA, Brazil
3Gonçalo Moniz Institute/Oswaldo
Cruz Foundation, BA, Brazil
Received:
January 6, 2021; Accepted: March 31, 2021.
Correspondence: Hiole Gonçalves Nolasco, Vila
São Roque, 413 Campinas de Brotas 40276-140 Salvador BA
Hiole Gonçalves Nolasco:
hiolenolasco17.1@bahiana.edu.br,
Djeyne Silveira Wagmacker: djeyne.ferreira@adventista.edu.br
Jacqueline de Jesus Silva:
jacqueline.jesus@fiocruz.br
Amâncio José de Souza: amanciosouza@bahiana.edu.br
Ana Marice Teixeira Ladeia:
analadeia@uol.com.br
Abstract
Introduction: Obesity is a
multifactorial condition related to the increase of serum arginase, favoring
endothelial dysfunction. Physical exercise is a protective factor for
endothelial homeostasis. Thus, knowing whether the level of arginase is
modified by a physical exercise session is important. Objective: To
verify the level of arginase in women with excess weight before and after a
physical exercise session and evaluate the association between the level of
arginase and the BMI of these patients. Methods: Exploratory study,
which used the serum bank of a randomized clinical trial, in which sedentary
women 18 to 30 years old, BMI > 24.9 kg/m² were included. Women with
diseases that could alter the levels of arginase were excluded. The volunteers
were randomized to the intervention group - a light intensity exercise session
(EG) and a control group (CG). The serum arginase of 11 women in the CG and 9
in the EG was measured before and 24h after the intervention. Data analysis was
performed using the SPSS 20 program using the unpaired Student's t-test and the
Mann–Whitney U test. Statistical significance is defined as p < 0.05. Results:
The patients' BMI was = 29 ± 4.7 kg/m². The intra-group analysis showed no
variation in serum arginase before and after the intervention, as well as in
the intergroup comparison using the arginase delta. There was no correlation
between the level of arginase and BMI. Conclusion: A session of physical
exercise did not change the levels of serum arginase in overweighted women.
Keywords: obesity; physical exercise;
arginase.
Resumo
Introdução: A obesidade é uma condição
multifatorial apresentando relação com a elevação de arginase
sérica, favorecendo a disfunção endotelial. O exercício físico é fator protetor
para homeostase do endotélio. Assim, conhecer se o nível de arginase
é modificado por uma sessão de exercício físico é de suma importância. Objetivo:
Verificar o nível de arginase sérica em mulheres com
excesso de peso antes e após uma sessão de exercício físico e avaliar a associação
entre o nível de arginase sérica e o IMC dessas
pacientes. Métodos: Estudo exploratório, que utilizou a soroteca de um ensaio clínico randomizado, no qual mulheres
sedentárias com idade entre 18 e 30 anos, IMC > 24,9 kg/m² foram incluídas.
Excluídas mulheres com doenças que pudessem alterar os níveis de arginase. As voluntárias foram randomizadas para grupo
intervenção - uma sessão de exercício de leve intensidade (GE) e grupo controle
(GC). A arginase sérica de 11 mulheres do GC e 9 do
GE foi dosada, antes e 24h após a intervenção. Análise dos dados foi realizada
no programa SPSS 20 através do teste t de Student não
pareado e Teste de Mann-Whitney. Significância estatística definida como p <
0,05. Resultados: O IMC das pacientes foi = 29 ± 4,7 kg/m². A análise
intragrupo não demonstrou variação da arginase sérica
antes e após a intervenção, bem como na comparação intergrupo através do delta
de arginase. Não se observou correlação entre nível
de arginase e IMC. Conclusão: Uma sessão de
exercício físico não modificou os níveis de arginase
sérica em mulheres com excesso de peso.
Palavras-chave: obesidade; exercício físico; arginase.
Introduction
Obesity,
according to the World Health Organization (WHO), can be understood as a
condition of multifactorial origin (historical, ecological, economic, social,
cultural, political, and genetic), resulting from a positive energy balance,
favoring the accumulation of fat and adding health
risks due to its metabolic implication in blood pressure, cholesterol levels,
serum triglycerides and insulin resistance [1,2].
Overweight and
obesity are found with high prevalence, since the age of 5, in all Brazilian
regions [3]. According to data from Vigitel 2017, 53%
of women in Salvador are overweight (BMI ≥ 25 kg/m²), and 20.4% are obese
(BMI ≥ 30 kg/m²) [4]. Until the end of 1980, this condition was growing
modestly, but tripled in the last 20 years and, in the last 6 years, the
increase is higher than 1% per year. The intake of high energy density foods
and the non-use of these calories by the human body are essential parts of the
metabolic imbalance, denoting the importance of both food reeducation and
physical exercise [3].
The increase in
the body mass index is related to the serum elevation of arginase [5], an
enzyme that is responsible for the hydrolysis of L-arginine, the principal
substrate for the formation of nitric oxide (NO) [6,7,8,9]. NO has many functions
in the body, including its contribution to endothelial relaxation [10]. Once in
a reduced quantity, as in the overweight population, there will be endothelial
dysfunction due to the absence of nitric oxide, in addition to an increase in
vasoconstrictor factors [11,12].
In contrast,
physical exercise, both acute and chronic, increases NO concentration,
promoting positive adjustments in the cardiovascular, hepatic, skeletal muscle
systems, among others [10]. This increase occurs through several mechanisms,
but most of the studies that explain them were carried out in animal models,
such as those carried out by Lee-Young et al. [13], Chies et al.
[14], Faria et al. [15], and Long et al. [16]. Thus, it
becomes necessary to investigate the topic in a more specific population of
human beings, searching for both biomolecular and genetic markers [10].
Since arginase
is an enzyme that is elevated in obese individuals and is related to the
increase in morbidity and mortality because of cardiovascular diseases due to
increase in total peripheral resistance in consequence of deficient
endothelium-dependent vasodilation, in addition to the scarcity of studies in
humans, knowledge of their levels in this population before and after an
exercise session is necessary. Thus, this study aimed to verify the level of
serum arginase in women with an excess weight under the acute effect of
physical exercise, as well as to evaluate the association between the level of
basal serum arginase and the BMI in overweighted women.
Methods
Population and study design
This study used
the serum bank of a randomized clinical trial, registered in the Clinical Trial
under the protocol NCT03170973, with an accessible population of the School-Clinic at Faculdade Adventista da Bahia, Cachoeira/BA,
Brazil. It was submitted to the Research Ethics Committee of Faculdade Adventista da Bahia,
approved under protocol 34017514.5.0000.0042. The collections were performed
from September 2015 to May 2016, and throughout the study, the guidelines on
research with human beings, Resolution 466/2012 of the National Health Council,
were observed.
The study
population that generated the serum bank was 66 volunteers, selected randomly
and invited to participate in the study. The inclusion criteria used were: female gender, 18 to 30 years old, BMI > 24.9
kg/m², and physical inactivity. The latter determined based on the
International Physical Activity Questionnaire - long version [17]. The
exclusion criteria were: women with parenchymal
cardiovascular, metabolic, and renal diseases, hypothyroidism or diabetes
mellitus, history of alcoholism or smoking, lipid-lowering drugs,
corticosteroids, diuretics, beta-blockers, and contraceptives usage.
Sample selection and intervention protocol
The group of 66
women selected according to these criteria was randomly divided, based on a
draw, into two groups, exercise, and control, both with 33 volunteers. For this
study, considering its initially exploratory character, about one-third of the
initial samples of each group were selected at random. In the exercise group,
the first blood collection occurred after 12 hours of fasting in the
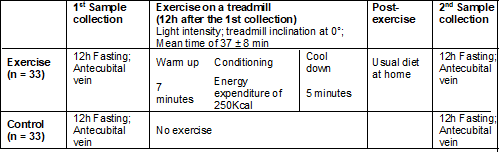
antecubital vein. After 12 hours of this collection, the patients underwent a
physical exercise session on a treadmill, divided into 3 times: warming up,
conditioning, and cooling down. The warm-up was 7 minutes, the conditioning
time corresponded to the energy expenditure of 250 kcal [18], and the cool-down
lasted 5 minutes, with the participants' average time in the physical activity
being 37 ± 8 min.
The intensity
control was determined by speed, the treadmill inclination was maintained at 0º
throughout the activity, and the intensity used was light, based on Borg's
perception of effort [19], that is, on the original scale corresponded to a
value between 9 and 11. For a better understanding of this scale, anchoring was
carried out before the day of the exercise, accustoming the volunteers to
respond appropriately when asked about the exercise intensity.
A polar
cardio-frequency meter was used, which measured energy expenditure based on
body mass, sex, and age, and after the exercise session, they were instructed
to return home and maintain their usual diet. After fasting for 12 hours, they
returned to the laboratory to collect the post-exercise blood sample, with a
24-hour interval between collections. The women in the control group were
submitted to the same protocol as the experimental group, but they did not
perform the exercise in the two previous days or 12 hours after the first and
second collections, respectively. The blood collection and exercise protocol
are shown in Chart 1, described in the control and exercise groups.
Chart 1 - Blood
collection and exercise in the Exercise and Control groups
The patients,
both in the exercise group and in the control group, were evaluated regarding
the diet of the day before the exam using a 24-hour food record, made through
an interview conducted at the time of blood collection. The volunteers reported
what they had consumed the previous day in and between the three main meals.
Serum samples storage and arginase dosage
The samples
consisted of 5mL of blood in tubes with EDTA, centrifugation at a speed of
3,000 rotations/min for 10 minutes after collection; the serum was aliquoted
and frozen at -80 ºC for further analysis. Among the 66 patients, 11 patients
in the control group (17, 40, 45, 35, 46, 47, 42, 12, 33, 51, and 57) and 11
patients in the exercise group (24, 36, 30, 50, 20, 58, 56, 28, 64, 66, and 7)
were randomly selected by the sample number. However, at the time of dosing,
hemolysis was observed in samples 24 and 36. Serum arginase dosage was
performed on the plasma samples at the NUPS Laboratory (Health Research Center)
of the Escola Bahiana de Medicina
e Saúde Pública.
The kit used was
an enzymatic immunoassay, sandwich ELISA, which has high sensitivity and
excellent specificity for detecting arginase. The duplicate method was used for
the arginase curve. The serum samples were separated previously for thawing
while the solutions were prepared. First, the standard solution was
reconstituted with 1.0mL of standard diluent (SD), with a concentration of
1.600 ng/mL. This was then diluted to 200 ng/mL and
is considered the highest standard [20]. Then, 7 tubes containing 0.5 mL of the
SD were separated for a serial dilution starting from the sample with 200 ng/mL
(tube 1 to tube 7) and the last one with 0 ng/mL (tube 8). The concentrations
obtained are described in Chart 2. Two detection reagents, A and B, diluted 100
times with their respective diluents were used. The washing solution (WS) was
diluted in 10 mL to 190 mL of distilled water, resulting in 200 mL of WS [20].
Chart 2 - Dilution
concentrations

For analysis, 14
wells were separated to place the standard solution, 2 for the blank, and 80
for the patient samples, as shown in Figure 1. 100 µl of the respective
substances were placed in each well, covered with the sealer, and incubated for
1 hour at 37°C. The liquid was removed but without washing. Then, 80 µl of
detection reagent A was added to each well, and after closing with sealer, it
was incubated for another 1 hour at 37°C. In this step, the washing was
performed with 350 µl of WS waiting 1-2 min and drying with absorbent paper,
repeating the process 3 times [20].
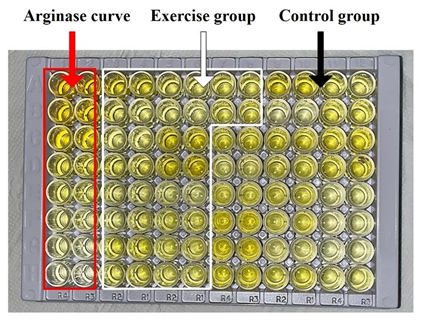
Figure 1 – ELISA
plate kit for detecting arginase filled with the curve and samples from the
exercise and control groups
Then, 80 µl of
detection reagent B was added to each well, closing again with the sealer and
incubating for another 30 minutes at 37°C. New washings were performed 5 times.
The substrate solution was added at a concentration of 90 µL in each well,
covered with a new sealer, and incubated for 10 to 20 minutes at 37ºC. After
the liquid turned blue [20], 50 µL of stop solution was added to the wells, and
the color changed to yellow. There were no water droplets or fingerprints on
the underside of the plate, as well as bubbles on the liquid surface. Once this
was done, the measurement was performed at 450 nm [20].
Statistical analysis
The continuous,
parametric variables were described as means and standard deviations and
compared using the Student's t-test for paired
samples. To verify the ELISA test's reliability, the mean and standard
deviation of the arginase curve was used, with R² = 0.9932.
The levels of
intragroup arginase have been described in medians and interquartile ranges.
For intergroup analysis, the arginase delta (baseline moment after
intervention) was used. The Mann–Whitney U test compared the intragroup
analysis' medians and associated the arginase levels with the patients' BMI,
these tests being used for the analysis of non-parametric variables.
All analyzes
were performed using SPSS (Statistical Package for the Social Sciences) version
20, Excel (2010 version), adopting a significance level with a value of p <
0.05.
Results
The study
included a serum bank of 20 women, 11 from the control group and 9 from the
exercise group, chosen at random. The mean age was 24 ± 3.4 years, and the BMI
= 29 ± 4.7 kg/m² in the general population. Subdividing the groups, the mean
age was 24.9 ± 3.7 years old and 24.2 ± 3.1 years old in the control and
exercise groups, respectively, with a p-value of 0.365. The mean BMI varied
between 30.2 ± 5.1 kg/m² in the control group and 28.9 ± 4.6 kg/m² in the
exercise group, with a p-value of 0.829, with no statistical significance. The
variable laboratory values (total cholesterol, HDL, LDL, triglycerides, blood
glucose, insulin, Homa-IR, and Homa-Beta)
did not differ between groups. The clinical characteristics are described in
Table I.
Table I - Laboratory
characteristics of overweight women in the total sample and by group on the 1st
day of blood collection
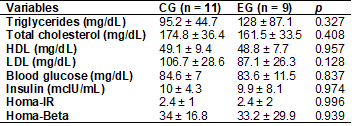
CG = Control group; EG
= Exercise group; p – Student's t-test
The arginase
curve obtained by diluting the standard solution in 8 samples of different
concentrations serves as a basis for the analysis of the others. It is possible
to observe in Table II and Graph 1, the mean and standard deviation of the
curve, the reliability of the test being notorious, since the curve follows a
linear and increasing pattern, with minimal deviation from the axis and with R²
= 0.9932, thus configuring a method of good sensitivity.
Table II – Arginase
curve
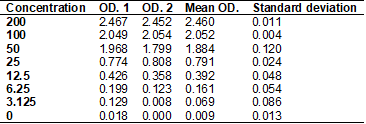
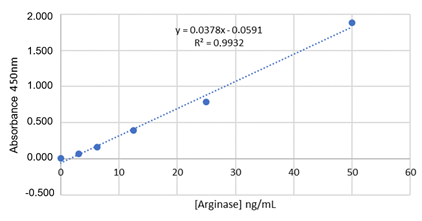
Graph 1 - Arginase
curve
The analysis of
the comparison of the intra-group arginase level on the first and second day of
the collection did not show variation in the concentration of serum arginase in
the experiment group, as well as in the control group, as described in Table
III and shown in Graphs 2 and 3.
Table III - Intragroup
analysis of serum arginase values in overweight women on the first and second
days

*Median (Interquartile
range). Mann–Whitney U test.
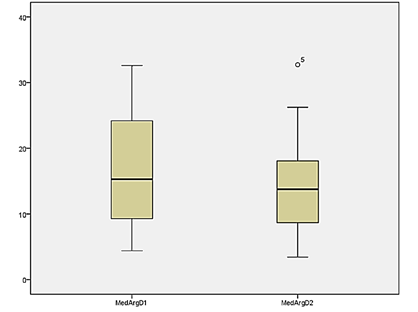
Graph 2 - Serum
arginase levels on the first and second day of collection in the control group
in overweight women
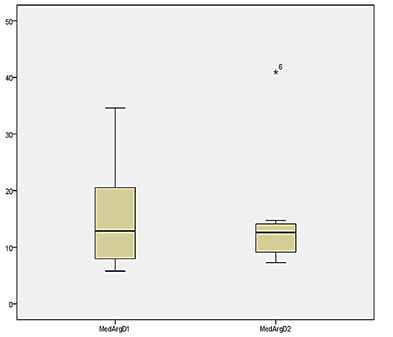
Graph 3 - Serum
arginase level before (first day) and after (second day) of an exercise session
in overweight women
In the
intergroup comparison, the variation between the baseline and post-intervention
moment (D
Arginase) of each of the groups was used, with no difference in the levels of
arginase between the control group and the exercise group [-0.99 (-7.9 - 4.7)
vs. 0.53 (-5.3 - 6.5); p = 0.71].
No association
was observed between the levels of arginase and the BMI of the patients,
separating them into overweight (n = 12) and obesity (n = 8), with an average
of arginase of 14.76 (SD = 9.89) and 20.96 (SD = 11.87) respectively, with
p-value = 0.436.
Discussion
This study's
results demonstrated that the performance of a single session of light physical
exercise in overweight women did not alter the levels of serum arginase.
A study with a
similar objective, however, with 41 individuals without a previous history of asthma,
diabetes mellitus, and other comorbidities, showed that after continued
exposure to moderate-intensity physical exercise (one hour of exercise
bike/day, 5x/week, for one month) the level of NO increased while L-arginine
was reduced, suggesting a direct relationship between physical exercise and improved endothelial function [21]. However,
when dosing arginase, in agreement with this study, it did not change between
pre- and post-intervention, believing that there was a nitrosylation
of the molecule, with activity regulated by the increase in NO [21]. It is
worth mentioning that no other studies were found that analyzed arginase before
and after exposure to physical exercise, being it in a single session or
continuously. Thus, it is not possible to make other comparisons.
Arginase is an
intracellular enzyme present in erythrocytes, liver, and kidneys, and is found
in plasma mainly after inflammation, chronic organ damage, and hemolysis [22].
Inflammatory markers such as TNF-α and CRP can contribute to the induction
of its activity [23], and in the overweight population, this component is
relevant.
White adipose
tissue, one of the constituents of adipose tissue, has 40% of macrophages in
its composition, being a contributing factor to the increased availability of
arginase in plasma since this enzyme is also induced by monocytes [24,25]. In
addition, it is suggested that at the time of weight gain, hypertrophy of
adipocytes occurs, followed by a mechanism of ischemia in the vessels of the
adjacent region and hypoxia, triggering a local inflammatory process and
chemotaxis of more macrophages to that region, promoting the elevation of TNF-α,
IL-6 and CRP [25]. In this same population, oxidative stress also contributes
to the inflammatory cascade [25], adding then one more factor that has been
proven to be related to the activation and increased expression of arginase
[5].
Other conditions
that contribute to the upregulation of arginase are insulin resistance and
liver damage secondary to non-alcoholic fatty liver disease, very common in the
overweight population [5]. These factors may justify the elevated baseline
serum levels of arginase in this population and are not amenable to reduction
with a single session of light physical exercise.
The increase of
this enzyme in this population of young women can also be detected in very
young individuals. For example, when compared to a population of adolescents
within normal weight and overweight, the average of arginase was 39.3 ± 26.9
ng/mL and 95.8 ± 68.2 ng/mL, respectively. In opposition to this study, there
was an association between arginase and anthropometric markers such as weight,
BMI, waist-to-hip ratio, and waist circumference, in addition to a family
history of arterial hypertension, CRP, and TNF-α, which strengthens the
biological plausibility of triad, obesity, inflammation and increased arginase
[26].
When comparing
non-obese and obese rats, the elevated serum arginase
and the development of endothelial dysfunction in those overweight were well
established, proving that the competitive relationship between arginase and NO
by the substrate promotes deficient NO-mediated vasodilation in this
population. When administered with arginase or arginine inhibitors, the
substrate itself, the endothelial response was considerably more satisfactory
[4].
Given this
competitive relationship and although it is proven that physical exercise is
associated with the preservation of the functional capacity of the endothelium
by promoting an increase in NO concentration after a single session [10], it is
not possible to establish its impact with the reduction of levels of arginase
without comparison with the variations that may occur in the serum level of NO.
It is important
to note that the arginase's role in endothelial dysfunction in this population
is due to its chronic maintenance at high levels. Faced with this, physical
exercise promotes an immediate compensatory response from the body, demanding
an increase in NO, which happens, even if in an imperfect way. The measurement
of NO in this study would allow us to compare the endothelial response with the
levels of arginase in patients in the exercise group, demonstrating how much
this competition for the substrate could interfere with endothelial
dysfunction.
Although there
is scientific evidence in the literature that establishes a correlation between
increased BMI and increased arginase, the present study did not find
statistical significance when comparing these data. The study had some
limitations that may have interfered with the results, such as small sample,
low intensity and frequency of exercise, and absence of direct assessment of
the NO level, as well as the evaluation of other factors that could potentially
alter the enzyme, such as inflammation, liver damage, and insulin resistance.
However, as this is a random sample, these possible biases are attenuated, and
our results are not infeasible.
Conclusion
In this
exploratory study, a single session of physical exercise was not able to modify
the levels of serum arginase in overweight women.
References
- Brasil.
Ministério da Saúde.Secretaria de Atenção à Saúde.
Departamento de Atenção Básica. Estratégias para o cuidado da pessoa com doença
crônica: obesidade. Available from:
http://bvsms.saude.gov.br/bvs/publicacoes/estrategias_cuidado_doenca_cronica_obesidade_cab38.pdf
- Cercato C,
Fonseca FA. Cardiovascular risk and obesity. Diabetol
Metab Syndr
2019;11(1):1–15. Available from: https://doi.org/10.1186/s13098-019-0468-0
- Brasil.
IBGE. Ministério do Planejamento, Orçamento e Gestão. Pesquisa de Orçamentos
Familiares 2008-2009. Análise do consumo alimentar pessoal no Brasil. Rio de
Janeiro. 2011. Available from:
https://biblioteca.ibge.gov.br/visualizacao/livros/liv50063.pdf
- Vigitel.
Vigitel Brasil 2017 - Vigilância de fatores de risco
e proteção para doenças crônicas por inquérito telefônico. Brasília
(DF); 2018. 1ª ed. Available from:
https://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2017_vigilancia_fatores_riscos.pdf
- Johnson F, Peyton K, Lui X, Azam M, Shebib A, Johnson R, et al. Arginase promotes endothelial
dysfunction and hypertension in obese rats. Obesity (Silver Spring)
2015;23(2):383-90. doi: 10.1002/oby.20969 [Crossref]
- Durante W, Johnson F, Johnson R. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol 2007;34(9):906-11. doi: 10.1111/j.1440-1681.2007.04638.x [Crossref]
- Pereira
NR. A via L-arginina – óxido nítrico, estresse
oxidativo e ciclo da uréia na obesidade [Tese]. Rio
de Janeiro: Universidade Estadual do Rio de Janeiro; 2011.
- Ogino
K, Takahashi N, Takigawa T, Obase
Y, Wang DH. Association of serum arginase i
with oxidative stress in a healthy population. Free Radic
Res 2011;45(2):147–55. doi: 10.3109/10715762.2010.520318 [Crossref]
- Momma TY, Ottaviani JI. Arginase inhibitor, Nw-hydroxy-L-norarginine,
spontaneously releases biologically active NO-like molecule: Limitations for
research applications. Free Radic Biol Med 2020;152:74-82. doi: 10.1016/j.freeradbiomed.2020.02.033 [Crossref]
- Souza Junior TP, Asano RY, Prestes J, Sales MPM, Oliveira Coelho JM, Simões HG. Óxido nítrico e exercício: Uma revisão. Rev da Educ Fis 2013;23(3):469-81. doi: 10.4025/reveducfis.v23i3.11738 [Crossref]
- Zimmer
A. Efeitos do treinamento físico aeróbio sobre o metabolismo do óxido nítrico e
da endotelina-1 e sobre o estresse oxidativo no parênquima pulmonar de ratos
com hipertensão arterial pulmonar [Dissertação]. Porto
Alegre: Universidade Federal do Rio Grande do Sul; 2016. Available
from: https://lume.ufrgs.br/handle/10183/157938
- Clemente GS, van Waarde A, Antunes IF, Dömling A, Elsinga PH. Arginase as a potential biomarker of disease progression: A molecular imaging perspective. Int J Mol Sci 2020;21(15):1-36. doi: 10.3390/ijms21155291 [Crossref]
- Lee-Young RS, Ayala JE, Hunley CF, James FD, Bracy DP, Kang L, et al. Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am J Physiol Regul Integr Comp Physiol 2010;298(5):1399-408. doi: 10.1152/ajpregu.00004.2010 [Crossref]
- Chies AB, de Souza Rossignoli P, Daniel EF. Exercise increases the angiotensin II effects in isolated portal vein of trained rats. Peptides 2010;31(5):883-8. doi: 10.1016/j.peptides.2010.02.011 [Crossref]
- Faria TDO, Targueta GP, Angeli JK, Almeida EAS, Stefanon I, Vassallo DV, et al. Acute resistance exercise reduces blood pressure and vascular reactivity, and increases endothelium-dependent relaxation in spontaneously hypertensive rats. Eur J Appl Physiol 2010;110(2):359-66. doi: 10.1007/s00421-010-1508-5 [Crossref]
- Long X, Bratz IN, Alloosh M, Edwards JM, Sturek M. Short-term exercise training prevents micro- and macrovascular disease following coronary stenting. J Appl Physiol 2010;108(6):1766-74. doi: 10.1152/japplphysiol.01014.2009 [Crossref]
- Matsudo S, Araujo T, Matsudo V, Andrade D, Andrade E,
Oliveira LC, et al. Questionario Internacional de atividade fisica (I PAQ): estudo de validade e reprodutibilidade no
Brasil. Revista Brasileira de Atividade Física & Saúde - RBAFS 2012;6(2). Available from:
https://rbafs.org.br/RBAFS/article/view/931
- Plaisance EP, Mestek ML, Mahurin AJ, Taylor JK, Moncada-Jimenez J, Grandjean PW. Postprandial triglyceride responses to aerobic exercise and extended-release niacin. Am J Clin Nutr 2008;88(1):30-7. doi: 10.1093/ajcn/88.1.30 [Crossref]
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg rating of perceived exertion scale in healthy individuals. J Sports Sci 2002;20(11):37-41. doi: 10.1080/026404102320761787 [Crossref]
- Cloud-Clone Corp. ELISA Kit for Arginase. 2013;1:2-9. Available from:
https://www.cloud-clone.us/elisa/ELISA-Kit-for-Human-Arginase-Arg-902.htm
- Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino O. Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr 2017;60;(3):180-6. doi: 10.3164/jcbn.16-108 [Crossref]
- Santos
W. Metabolismo da arginina e moléculas associadas a ativação endotelial na
anemia falciforme [Tese]. Salvador: Universidade Federal da Bahia/ Fundação
Oswaldo Cruz Salvador; 2011. Available from: https://www.arca.fiocruz.br/handle/icict/4323
- Maritza JR, Daniel HP, Huda ET, Mohamed L, Azza BE, Manuela B, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 2008;102(1):95-102. doi: 10.1161/CIRCRESAHA.107.155028 [Crossref]
- Fruzsina KJ, Kelly JP, Xiao-ming L, Mohammed AA, Ahmad RS, Robert AJ, et al. Arginase promotes endothelial dysfunction and hypertension in obese rats. Obesity (Silver Spring) 2015;23(2):383-90. doi: 10.1002/oby.20969 [Crossref]
- Leite
L, Rocha E, Brandão-Neto J. Obesidade: uma doença inflamatória. Revista Ciência
& Saúde 2009;2(2):85-95. Available from:
https://www.researchgate.net/publication/277162907_Obesidade_uma_doenca_inflamatoria
- Jung C, Figulla HR, Lichtenauer M, Franz M, Pernow J. Increased levels of circulating arginase I in overweight compared to normal weight adolescents. Pediatr Diabetes 2014;15(1):51-6. doi: 10.1111/pedi.12054 [Crossref]