Rev Bras Fisiol Exerc 2021;20(3):398-412
doi: 10.33233/rbfex.v20i3.4562
REVIEW
Exercise-induced or exacerbated immune and allergic
syndromes: what the exercise health professional needs to know about immunoallergic diseases and sport?
Síndromes
imunológicas e alérgicas induzidas ou exacerbadas por exercício: o que o
profissional de saúde do exercício precisa saber sobre doenças imunoalérgicas e esporte?
Sérgio
Dortas Duarte Júnior1, Guilherme Gomes
Azizi1
1Universidade Federal do Rio de Janeiro,
Rio de Janeiro, RJ, Brazil
Received:
January 21, 2021; Accepted:
June 21, 2021.
Correspondence: Guilherme Gomes Azizi, Rua
Prof. Rodolpho Paulo Rocco, 255, 9°andar, Sala 9E10, Cidade Universitária da
Universidade Federal do Rio de Janeiro, 21941-617 Rio de Janeiro RJ, Brazil
Sérgio Dortas Duarte Júnior:
sdortasjr@gmail.com
Guilherme Gomes Azizi:
gazizi247@gmail.com
Abstract
Exercise-induced or exacerbated immunoallergic
diseases are significantly important situations for both amateur and professional
athletes. Asthma, bronchospasm, exercise-induced laryngospasm
and anaphylaxis, chronic inducible urticaria, and hereditary angioedema are
examples of these situations. This article aims to contribute to the knowledge
of health professionals with guidance on the diagnosis and management of
hypersensitivity disorders induced by exercise or triggered during sports
practice, to allow their patients to safely perform activities related to
exercise.
Keywords: asthma; anaphylaxis; angioedema; exercise;
urticaria
Resumo
As
doenças imunoalérgicas induzidas ou exacerbadas pelo
exercício são situações significativamente importantes tanto para atletas
amadores quanto profissionais. Asma, broncoespasmo, laringoespasmo
e anafilaxia induzidos pelo exercício, urticárias crônicas induzidas e
angioedema hereditário são exemplos destas situações. O objetivo deste artigo é
contribuir para o conhecimento de profissionais de saúde com orientação ao
manejo de distúrbios de hipersensibilidade induzidos por exercícios ou desencadeados
durante a prática esportiva, para permitir que seus pacientes realizem, com
segurança, as atividades relacionadas ao exercício.
Palavras-chave: asma; anafilaxia; angioedema;
exercício; urticária
Exercise-induced
or exacerbated immune allergic diseases are significantly important situations
for both amateur and professional athletes. Asthma, bronchospasm,
exercise-induced laryngospasm and anaphylaxis,
inducible urticaria, and hereditary angioedema are examples of these
situations. This article aims to contribute to the knowledge of health
professionals with guidance on the diagnosis and management of hypersensitivity
disorders induced by exercise or triggered during sports practice, to allow
their patients to safely perform activities related to exercise.
Exercise-induced asthma/bronchospasm
Exercise-Induced
Bronchospasm (EIB), formerly called “Exercise-Induced Asthma”, is defined as
the transient narrowing of the airways in response to a wide variety of
bronchoconstrictor stimuli related to intense physical exercise, presenting
symptoms such as coughing, dyspnea, and wheezing. This condition occurs in a
subgroup of individuals with asthma and in some non-asthmatics [1,2,3]. Thus,
demonstrating a characteristic of intense airway hyperresponsiveness, EIB is
more common in winter sports athletes and swimmers than in general population
and athletes from other sports.
The prevalence
in the Brazilian population was analyzed in 2 studies from different regions, Recife and São Paulo. Both demonstrated that children and
adolescents with asthma have a prevalence of about 45% of EIB [4,5].
The intensity of
ventilation, a fundamental factor for adequate oxygen supply during physical
activity, can also be the "Achilles tendon"
in individuals subject to EIB, as we can go from 6 L/min of respiratory volume
to more than 200 L/min. In addition, breathing becomes progressively oral, from
the moment the individual reaches 30 L/min.
Thus, mouth
breathing does not have the range of mechanisms present during adequate nasal
breathing, in which there is humidification and air heating, in addition, with
greater flow, there is greater exposure to aeroallergens, irritants to the
mucosa and particulate matter, which in the long term, can participate in the
pathophysiology of respiratory diseases such as asthma and mixed rhinitis
[6,7].
The
pathophysiology of EIB is not yet fully described, however, studies indicate
that there is possibly a correlation between airway cooling through inhaled air
and subsequent rewarming of the airways after exercise [8]. Another proposed
hypothesis is related to airway dehydration air, which through the intensity of
ventilation, results in an increase in the osmolarity of the local fluid,
increasing the periciliary movement and, consequently, increasing the water in
the bronchial lumen [9]. Thus, it would release inflammatory mediators leading
to bronchoconstriction through the contraction of smooth and edema [10].
Exercise immunology could explain the third hypothesis for this multivariate
pathology, since high-performance athletes go through periods of transient
immunosuppression called “Open Window”, in which they are more susceptible,
especially which can exacerbate symptoms pre-existing conditions or cause
isolated bronchoconstriction [11,12,13].
The diagnosis of
asthma can be made based on a history of characteristic symptoms (cough,
wheezing, chest pain, and dyspnea) and documentation of variable airflow
limitation, by means of spirometry with bronchodilator testing or
bronchoprovocation tests, as the clinical diagnosis in the EIB can be
complicated [14]. The diagnosis of EIB uses the variation in FEV1 before and in
sequences of 5, 10, 15, and 30 minutes after the provocation tests, through
vehicles such as treadmills or stationary bicycles. Forced expiration maneuvers
should be performed in a standardized way, and the calculation of the variation
should be performed in relation to the baseline value, with a reduction in FEV1
> 10% or 15%, which are observed in one or two moments of the assessment,
depending on the literature [14]. To carry out the provocation test, the
athlete should not practice any exercise in the previous 4 hours, as this could
lead to a false-negative result, due to refractoriness in this period [1,16].
Differential
diagnoses to EIB must always be considered, such as exercise-induced
laryngospasm, poorly controlled rhinitis, gastroesophageal reflux, and
hyperventilation syndrome. The goals of asthma treatment are to achieve and
maintain asthma control, improve lung function, and prevent risk factors for
acute events such as exacerbations. Specifically, in relation to the EIB, it
will directly depend on the correlation with asthma or not [17,18].
Environmental
measures and masks can help reduce the effects of exposure to cold air on
winter sports athletes or the inhalation of air pollutant particles [19].
In addition to
these, the pre-exercise warm-up can result in a reduction in
bronchoconstriction by exercise in about 50% of individuals, which is performed
for at least 10 to 15 minutes, reaching up to 60% of the maximum heart rate.
Then, the athlete will enter a "refractory period" induced by the release
of protective prostaglandins [20].
There are few
randomized clinical trials for an adequate analysis of pharmacotherapy for the
treatment of EIB. However, inhaled glucocorticoids are the mainstay of therapy
for asthma, as this is basically a pathology with inflammatory characteristics
[16], these agents, by inhalation, are allowed by sports authorities, such as
the World Anti-doping Agency (WADA) and the Authority Brazilian Association of
Doping Control (ABCD) [22,23].
The most
commonly used strategy for athletes with or without asthma and who have EIB is
treatment with inhaled glucocorticoids, inhaled b2-agonists before exercise (regular or if necessary)
in association or not with b2
receptor antagonists leukotrienes (montelukast)
[1,23].
Long-acting b-agonists are good options for athletes, as they have
a bronchodilator action of up to 12 hours, unlike salbutamol, the main
short-acting b-agonist
with an action of up to 3 hours [18]. Formoterol and salmeterol (b-agonists of long-lasting) have no WADA restriction.
The association with inhaled corticosteroids is increasingly present, leaving
the isolated prescription of b-agonists
in the past, as this interaction minimizes tachyphylaxis and favors
inflammation control [17].
Immunotherapy
for aeroallergens has limited effectiveness in direct relation to EIB, as there
are no large studies, however, immunotherapy is widely used in asthma or
allergic origin, so this possibility should be analyzed together with the
specialist, as in addition to being a modifying factor in the natural history
of the disease, it is not a treatment characterized as doping [17,21,24].
Thus, we must
emphasize that the health professional must act both for the health of the
individual and for the well-being of the individual's work instrument, their
body, since any reduction in physical capacity can be the line between victory
and defeat.
Exercise-induced laryngospasm
Exercise-induced
laryngospasm (EILs) are a group of conditions that cause laryngeal obstruction
during exercise, among these are exercise-induced laryngomalacia (EIL, a
supraglottic obstruction caused by arytenoid collapse) and exercise-induced
vocal cord dysfunction (CVIE) [25,26].
EILs have
symptoms like exercise-induced asthma and have a high prevalence among the
population, however, it is still confused with EIA, which causes a
misdiagnosis, however, many do not have associated asthma [27,28] from
approximately 5% to 7% among adolescents and young adults [29].
The supraglottic
obstruction appears to precede the inspiratory glottic narrowing, in greater
proportion than during the expiratory period [20]. This and other
anatomical-physiological factors provide some hypotheses that suggest that EIL
has varied etiologies, these being the size of the larynx, which could
contribute as a causal or facilitating factor, such as during puberty, when the
laryngeal diameter of women begins its process of reaching a smaller diameter
in relation to men, explaining the higher prevalence of adolescent women with a
report of EIL [30,31].
Another
etiological hypothesis involves the pressure difference during the increase in
intensity in a physical activity that normally requires accelerated breathing
movements. Thus, it would be a partially passive phenomenon, in which increased
effort and ventilation would increase the negative transmural pressure [32].
In addition, the
anterior movement of the epiglottis puts tension on adjacent structures and
would facilitate supraglottic closure, mainly due to the high tension of the
aryepiglottic fold, pulling the arytenoid mucosa anteriorly, reducing the
circumference of the larynx [33].
A third
hypothesis would be hypersensitivity of the upper airways, in a physiological
reflex of the glottic region to avoid aspiration, which could explain an
inadequate local adduction [33].
In addition to
these hypotheses, a fourth possibility was proposed for the origin of symptoms,
which would be closely related to gastroesophageal reflux, as after acid reflux
reaching the laryngopharyngeal area, it would induce a state of
hyperexcitability [34]. Thus, complementary diagnostic research is indicated
and propose treatment with proton pump inhibitors. We must remember that the
prevalence of reflux in the population varies between 10% and 60% [35].
Therefore, this would be a reasonably important hypothesis to be considered.
The management
of the EIL is still under wide discussion and without a defined consensus,
mainly due to the heterogeneity of the etiology and the possible phenotypes
involved. Thus, a careful evaluation is indicated, in which predisposing and
irritating factors that may develop the obstruction are excluded, in addition
to the exclusion of differential diagnoses, including exercise-induced
bronchospasm. Studies also associate psychological therapy as a complementary
factor in treatment [36,37].
Some reports
sought to identify possible therapies such as the use of inhaled ipratropium
bromide before the activity, which would reduce vocal cord dysfunction [38]. In
addition to the approaches described above, the possible surgical intervention
should be evaluated together with the otolaryngologist surgeon.
Exercise-induced anaphylaxis
Anaphylaxis is
derived from the Greek language ana ("inversion", "repeat")
and phylaxis ("guard", "immunity"), having been adopted by
Charles Robert Richet and Paul Portier in 1902 [38]. It is characterized by an
intense and potentially hypersensitivity reaction fatal that results from a
systemic release of inflammatory mast cell and basophilic mediators such as
histamine, leukotrienes, tryptase, often correlated with a reaction involving
immunoglobulin E (IgE) [38,39].
Anaphylactic
reactions have an intense correlation with some allergens common in our
environments, such as food, anti-inflammatory drugs, b-lactams, and insect venom (bees and wasps), due to
previous sensitization (specific IgE) [40]. However,
there are anaphylactic conditions in which the patient does not have
sensitization to the causative agent, as indirect mast cell degranulation after
MRGPRX2 receptor stimulation by drugs (quinolones, neuromuscular blockers, icatibant, opioids), mastoparan
wasp venom, and substance P [41,42].
The clinical
characterization of anaphylaxis is still not consensual. The clinical picture
starts about 5-30 minutes after exposure to the allergen, however, symptoms can
be observed within 6 hours.
Manifestations
generally occur with skin involvement associated with one or more of the
respiratory (70%), cardiovascular (10-45%), central nervous system (10-15%),
and gastrointestinal tract (30-45%) systems. However, possible anaphylaxis
should not be neglected if there is no skin involvement [42,43].
Chart I - Signs and
symptoms

Modified of
“Guia para manejo da anafilaxia-2012” – Grupo de Anafilaxia da ASBAI. Rev Bras Alerg Imunopatol
2012;35(2)
The differential
diagnosis of anaphylaxis must be considered, however, disregarding possible
anaphylaxis can lead to the patient's death. Thus, any affection that acts on
the skin and mucous membranes can cause laryngotracheitis, bronchial
obstruction, or an asthma exacerbation, as well as vasovagal syncope, pulmonary
embolism, and other emergencies in other systems correlated with anaphylaxis
[42].
Exercise-induced
anaphylaxis (EIA) is a condition initially reported by Maulitz
et al. [44] in 1979, which described a picture of hypersensitivity
occurring in vigorous physical activity preceded by ingestion of shellfish
(shrimp and oysters) between 5 and 24 hours before.
It is estimated
that EIA may have an incidence between 7 and 9% within the epidemiology of
anaphylaxis [45], and may occur at any intensity of physical activity, however,
studies have shown that sports with lower cardiovascular demand have fewer
reports [46].
Foods with the
greatest involvement in exercise-induced anaphylaxis with IgE-mediated
food dependence are wheat (correlated with 5-omega-gliadin), shellfish,
peanuts, corn, cow's milk, soy, mite-contaminated flours, and fruits from
Rosaceae family (peach, loquat, plum, apricot, cherry, and others) [44,47]. The
symptoms can start during or after exercise, however, most occur about 30
minutes after stopping the activity [44].
EIA may also
have medications as triggers, mainly non-steroidal anti-inflammatory drugs and antibiotics (cephalosporins), requiring the
interaction between medication and physical activity [44].
Several
hypotheses have been suggested to explain this disease. The most accepted
hypothesis would be the correlation of physical activity with increased
gastrointestinal permeability [44]. This pathophysiology would be related to
the increase in low-affinity IgE receptors in the
intestinal mucosa cells, which, in patients with food allergy, could stimulate
the cascade inflammatory potentiated by increased blood flow to physical
exercise. Studies have shown that exercise and ASA enhance the absorption of
allergens, especially the omega-5-gliadin present in wheat. Thus, if a food
challenge with exercise or AAS occurs or is purposely performed, it could
induce the anaphylactic manifestation [44,47].
Transglutaminase
could alter the absorption of food allergens, which in association with
exercise could accelerate the process of allergen distribution. Other triggers
described in the literature would be environments of high or low temperature,
high humidity, exposure to seasonal pollen, especially in northern hemisphere
countries, alcoholic beverages, stress, infection, and menstrual period [48].
The diagnosis is
entirely related to a good anamnesis. However, serum tryptase measurement after
the suspected or confirmed condition of exercise-induced anaphylaxis could
confirm this, as well as in anaphylaxis, enabling subsequent preventive
intervention regarding the allergen triggering the reaction. Thus, the
investigation of allergic sensitization to foods. However, if the history and
the search for sensitization (specific IgE) are not
clear, the challenge test will be an important tool.
There is no
standardized challenge test exclusively for EIA. However, the Bruce protocol is
a maximal exercise test that uses a treadmill and encourages an increase in
speed and incline every 3 minutes, so, due to its easy reproduction, this test
is widely used in association with the previous intake of said food causing the
reaction. We must remember that the environment must be controlled, vital signs
must be monitored continuously and the test must be
performed under medical supervision. The patient should be asked to discontinue
antihistamines and leukotriene antagonists for at least 3 days before the
challenge test [49,50,51].
After the
diagnostic hypothesis is proposed, the necessary support must be offered for
the well-being of the athlete or practitioner of physical activity. All
patients should be prescribed and trained to manage self-injecting epinephrine.
In addition, the patient must be educated about the characteristic symptoms of
anaphylaxis, its possible triggers (avoid food between 4-6h prior to exercise,
avoid aspirin and/or NSAIDs between 24 and 48h prior to activity) involved in
each case, and recommends the performance of physical activities always
accompanied. H1 antihistamines can and should be used according to the
symptoms, on a regular basis or before physical activity, if the specialist
deems it necessary [41,44].
Chronic inducible urticaria
According to
current guidelines, urticaria is defined as a condition determined by the onset
of urticaria, angioedema, or both. Wheals is characterized by a lesion with
central edema of variable size, almost always surrounded by erythema, a
sensation of itching or burning, and fleeting nature, with the skin returning
to its normal appearance between 30 minutes and 24 hours. Angioedema, in turn,
presents as sudden and pronounced edema of the lower dermis and subcutaneous
tissue, or mucous membranes, with a sensation of pain at the site, and slower
resolution than wheals, which may last up to 72 hours [52].
Urticaria is
classified according to the duration of clinical manifestations as acute when
signs and symptoms persist for less than 6 weeks, or chronic in cases where it
manifests daily or almost daily for 6 or more weeks. Chronic urticaria (UC), in
turn, can occur spontaneously or be induced by specific stimuli such as cold,
heat, pressure, increase in body temperature (cholinergic urticaria), etc.
[52].
Cholinergic
urticaria and cold urticaria are important situations to be considered in the
context of sports practice [53]. Cholinergic urticaria is characterized by the
appearance of micropapular lesions, related to an
increase in body temperature, from physical exercise or local application of heat; in addition to emotional stress, spicy foods or hot
drinks. The lesions are approximately between 1 and 3 mm, located on the trunk
and upper limbs. Lesions tend to last 15 to 60 minutes and may be associated
with local angioedema. If cholinergic urticaria is suspected, it is important
to differentiate it from exercise-induced anaphylaxis, aquagenic urticaria,
adrenergic urticaria, and cold-induced cholinergic urticarial [54,55].
The provocation
test to confirm cholinergic urticaria also aims to rule out exercise-induced
anaphylaxis. A standardized protocol for diagnosing and measuring cholinergic
urticaria thresholds using heart rate monitoring exercise testing has been
proposed. The test is performed by ergometry with heart rate control, so the
patient positions himself on the ergometric bicycle and starts pedaling, being
instructed so that the heart rate rises by 15 beats per minute every 5 minutes,
reaching 90 beats per minute above the basal level after 30 minutes. The time for
the onset of urticaria is inversely proportional to the intensity of the
disease (image 1), that is, the shorter the time for the onset of lesions, the
more severe the cholinergic urticaria is [54].
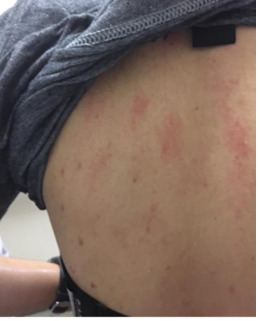
Image 1 - Lesions
compatible with cholinergic urticaria after provocation test. HUCFF-UFRJ Immunology Service
Courtesy
The therapy of
the first choice consists of non-sedating antihistamines. However, there are
alternatives for refractory cases such as omalizumab, an anti-IgE monoclonal antibody.
Cold urticaria
is defined by the appearance of wheals after exposure to cold, whether by solid
objects, air, or cold liquids. Lesions are usually limited to the site of
contact with cold (wheals and angioedema), but they can be generalized and
accompanied by systemic manifestations, including progression to acute
respiratory failure and anaphylaxis. These mainly occur in situations such as
carrying refrigerated objects, swimming in ice water, staying, or entering a
refrigerated environment, which can put swimmers and skiers at high risk
[52,56].
Challenge
methods for cold urticaria include the classic “ice cube test” (picture 2 and
2.1) and the TempTest® (picture 3 and 3.1). Management of cold urticaria includes: avoiding cold exposure, drinking or cold
foods; non-sedating antihistamines in recommended doses or even quadrupled; in
selected cases the use of omalizumab. In severe cases, with cold anaphylaxis,
an emergency plan must be instituted, including the prescription of epinephrine
autoinjectors, which is the gold standard medication in severe conditions
involving inflammatory mediators, such as histamine [54].
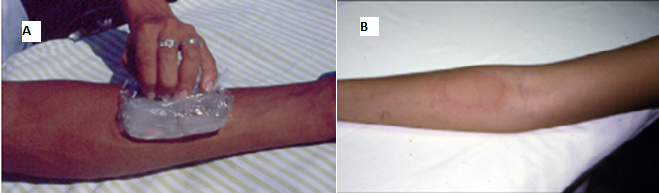
Image 2 - (A) - Ice cube
provocation test (Ice cube test) for diagnosis of cold urticaria; (B) -
Positive “ice cube” challenge test for cold urticaria. HUCFF-UFRJ Immunology
Service Courtesy
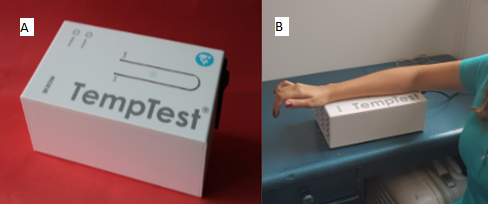
Image 3 – (A) Temp
Test® -Instrument aimed at provocation testing in cold urticaria and heat urtica-ria; (B) Carrying out the specific provocation test
for cold urticaria through TempTest®. HUCFF-UFRJ
Immunology Service Courtesy
Delayed Pressure
Urticaria (DPU) is a condition in which deep tissue swelling occurs several
hours after a sustained pressure stimulus, for example, wearing a mouthguard,
prolonged adherence to sports equipment, on the soles of the feet after
running, or on the buttocks after long-distance cycling or rowing. The
therapeutic response is variable to antihistamines and the use of quadrupled
doses is often necessary. Omalizumab, dapsone, sulfasalazine, anti-TNF, and
theophylline have also been used to control DPU symptoms [54,57,58].
Solar urticaria
occurs in individuals shortly after exposure to the sun. Management includes
barrier protection, use of sunscreens, and antihistamines before sun exposure.
Different therapeutic modalities were described, according to the intensity of
the symptoms: sunscreen, oral antihistamines, cyclosporine, desensitization
with different types of phototherapy, omalizumab,
plasmapheresis, Intravenous Immunoglobulin (IgIV), afamelanotide, among others. Although therapeutic
recommendations have been proposed in the context of chronic urticarias, there
are no consensus-based guidelines that define the specific approach for solar
urticarial [54,57].
Aquagenic
urticaria is uncommon and represents a body's reaction to water; this is
independent of temperature. H1 antihistamines and UV therapy are used to treat
this disease with variable response [54,57].
Hereditary angioedema
Hereditary
angioedema (HAE) is a rare, potentially fatal disease characterized by attacks
of cutaneous edema, submucosal and not correlated with wheals (image 5).
Patients with HAE have a quantitative or qualitative defect in the C1 inhibitor
(C1-INH), an enzyme from the SERPINA superfamily that acts as a serine
protease. Later a new group of HAE patients with normal C1-INH has been defined
[58].
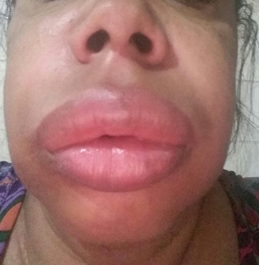
Image 4 - Large
angioedema in a patient with Hereditary Angioedema; UCFF-UFRJ Immunology
Service Courtesy
Three types of
HAE are defined: 1) HAE with quantitative C1-INH deficiency (formerly
designated as HAE C1-INH Type I); 2) HAE with C1-INH dysfunction (formerly
designated as HAE C1-INH of Type II); and AEH with normal C1-INH (formerly
referred to as AEH Type III) [58,59,60].
The main
mediator of angioedema in patients with HAE-1/2 is bradykinin through the binding
of this mediator to its B2 receptor, which is constitutively expressed in
endothelial cells and interferes with endothelial junctions, increasing
vascular permeability [61].
Patients with
HAE suffer from recurrent angioedema episodes involving the skin and submucosa
of various organs. The most affected sites are the face, extremities,
genitalia, oropharynx, larynx, and digestive system. However, rare clinical
manifestations such as severe headache, urinary retention, and acute pancreatitis
can also occur [61].
Although many of
the crises occur spontaneously, several triggering factors have been
identified: trauma (even if mild), stress, infection, menstruation, pregnancy,
alcohol consumption, extreme temperature changes, exercise, use of ACE
inhibitors, and use of estrogen (contraceptives and hormone replacement
therapy). In adolescence, there may be a substantial increase in disease
activity, particularly in young females, due to menstrual cycles and the use of
oral contraceptives containing estrogen. As trauma is among the main triggering
causes of crises, impact/combat physical activities should be discouraged for
these patients [61].
HAE can present
with non-anaphylactic edema of the upper airways, which can cause suffocation
and death in athletes, as reported in an undiagnosed patient who practiced
martial arts as well as his family members [61]. Pharmacological treatment for
anaphylaxis is ineffective and airway management should not be delayed. If not
diagnosed, mortality can reach 33% [62,63].
All patients
with suspected AEH-1/2 (ie, recurrent angioedema in
the absence of a known cause) should be evaluated for blood levels of C4,
C1INH, and C1INH function; and these tests, if abnormally low, should be
repeated to confirm the diagnosis.
Education and
guidance are the most important initial actions to avoid serious consequences
of HAE and to improve the quality of life of patients and their families.
Patients should receive written information that is relevant about the HAE,
including preventive measures and an action plan for crisis management [59].
Identifying and
eliminating triggers such as stress and trauma can reduce the risk of seizures.
High-impact sports and hobbies that are at risk of trauma are contraindicated,
as are medications that can induce or prolong an HAE crisis, such as ACE
inhibitors, Angiotensin II receptor blockers (ARB), estrogen-containing
medications, and gliptins. Patients who need contraception should only receive
progestins. Vaccination against hepatitis A and B is recommended, as blood
products can be used in the treatment of HAE, although there is no record of
infection by these viruses in patients who used the drugs currently available
[59].
HAE
pharmacotherapy is divided into three modalities: long-term prophylaxis,
short-term prophylaxis, and treatment of crises. As this article is a
bibliography for sports emergencies, we took a moment to discuss the treatment
of angioedema crises in these patients.
Conclusion
Physical
activities can trigger different illnesses (asthma, rhinitis, anaphylaxis,
urticaria, and hereditary angioedema) that impair performance. The early
diagnosis of immunoallergic disorders in athletes is important in order to
implement effective preventive measures and rescue strategies, allowing the
full performance of physical activities.
Potential conflict of
interest
No potential conflicts
of interest relevant to this article have been reported.
Financing source
There were no external
funding sources for this study.
Author contributions
Conception and design
of the research: Dortas-Jr
SD. Data collection: Dortas-Jr SD, Azizi
G. Data analysis and interpretation: Dortas-Jr SD, Azizi G. Statistical analysis: Not
applicable. Writing of the manuscript: Dortas-Jr SD,
Azizi G. Critical review of the manuscript for important intellectual content: Dortas-Jr SD, Azizi G
References
- Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell
KW, Hull JH, et al. An official American thoracic society clinical practice
guideline: Exercise-induced bronchoconstriction. Am J Respir Crit Care Med
2013;187(9):1016-27. doi: 10.1164/rccm.201303-0437ST [Crossref]
- Kippelen P, Anderson S. Pathogenesis of exercise induced bronchoconstriction. Immunol Allergy Clin N Am 2013;33:299‑312. doi: 10.1016/j.iac.2013.02.002 [Crossref]
- Langdeau JB, Turcotte H, Bowie DM, Jobin J, Desgagné P, Boulet LP. Airway hyperresponsiveness in elite athletes. Am J Respir Crit Care Med 2000;161:1479-84. doi: 10.1164/ajrccm.161.5.9909008 [Crossref]
- Correia MA Jr, Rizzo JA, Sarinho SW, Cavalcanti Sarinho ES, Medeiros D, Assis F. Effect of exercise-induced bronchospasm and parental beliefs on physical activity of asthmatic adolescents from a tropical region. Ann Allergy Asthma Immunol 2012;108(4):249-53. doi: 10.1016/j.anai.2012.01.016 [Crossref]
- Sano F, Solé D, Naspitz CK. Prevalence and characteristics of exercise induced asthma in children. Pediatr Allerg Pediatr Allergy Immunol 1998;9(4):181-5. doi: 10.1111/j.1399-3038.1998.tb00370.x [Crossref]
- Weiler JM, Anderson SD, Randolph C, Bonini S, Craig TJ, Pearlman DS, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol 2010;105(Suppl):S1-S47. doi: 10.1016/j.anai.2010.09.021 [Crossref]
- Fitch KD, Sue-Chu M, Anderson SD, Boulet S, Hancox RJ, McKenzie D, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s consensus conference, Lausanne, Switzerland, January 22-24, 2008. J Allergy Clin Immunol 2008;122:254-60. doi: 10.1016/j.jaci.2008.07.003 [Crossref]
- Bonsignore MR, Morici G, Vignola AM, Riccobono L, Bonanno a., Profita M, et al. Increased airway inflammatory cells in endurance athletes: What do they mean? Clin Exp Allergy 2003;33(1):14-21. doi: 10.1046/j.1365-2222.2003.01557.x [Crossref]
- Anderson SD, Kippelen P. Exercise induced bronchoconstriction: pathogenesis. Curr Allergy Asthma Rep 2005;5:116-22. doi: 10.31189/2165-6193-5.3.37 [Crossref]
- Helenius I, Haahtela T. Allergy and asthma in elite summer sport athletes. J Allergy Clin Immunol 2000;106(3):444-52. doi: 10.1067/mai.2000.107749 [Crossref]
- Pedersen BK, Ullum H. NK cell response to physical activity: possible mechanisms of action. Med Sci Sports Exerc 1994;26(2):140-6. doi: 10.1249/00005768-199402000-00003 [Crossref]
- Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC,
Fleshner M, et al. Position statement. Part one:
Immune function and exercise. Exerc Immunol Rev 2011;17:6-63
- Azizi GG, Orsini M, Dortas Júnior SD, Vieira PC, Carvalho RS, Pires CSR, et al. COVID-19 e atividade física: qual a relação entre a imunologia do exercício e a atual pandemia? Rev Bras Fisiol Exerc 2020;19(2supl):S20-S29. doi: 10.33233/rbfe.v19i2.4115 [Crossref]
- Dickinson J, McConnell A, Whyte G. Diagnosis of exercise-induced bronchoconstriction: eucapnic voluntary hyperpnoea challenges identify previously undiagnosed elite athletes with exercise-induced bronchoconstriction. Br J Sports Med 2010;45(14):1126-31. doi: 10.1136/bjsm.2010.072520 [Crossref]
- Subbarao P, Duong M, Adelroth E, Inman M, Pedersen S, O’Byrne PM, et al. Effect of ciclesonide dose and duration of therapy on exercise-induced bronchoconstriction in patients with asthma. J Allergy Clin Immunol 2006;117:1008-13. doi: 10.1016/j.jaci.2005.11.048 [Crossref]
- Fitch KD, Morton AR. Specificity of exercise in exercise-induced asthma. BMJ 1971;4:577-81. doi: 10.1136/bmj.4.5790.814-c [Crossref]
- Global Initiative for Asthma. [Internet] 2020. [cited
2020 Nov 15]. Available from: http://www.ginasthma.org
- Silva D, Couto M, Delgado L, Moreira A. Diagnosis and treatment of asthma in athletes. Breathe 2012;8(4):287-96. doi: 10.1183/20734735.009612 [Crossref]
- Millqvist E, Bengtsson U, Löwhagen O. Combining a beta2-agonist with a face mask to prevent exercise-induced bronchoconstriction. Allergy 2000;55:672-5. doi: 10.1034/j.1398-9995.2000.00558.x [Crossref]
- Elkins MR, Brannan JD. Warm-up exercise can reduce exercise-induced bronchoconstriction. Br J Sports Med 2013;47:657-8. doi: 10.1136/bjsports-2012-091725 [Crossref]
- World Anti-doping Agency. [Internet]. [cited 2020 Nov 13]. https://www.wada-ama.org
- Autoridade
Brasileira de Controle de Dopagem. [Internet]. [cited 2020 Nov
13]. https://www.gov.br/abcd/pt-br
- Duong M, Amin R, Baatjes AJ, Kritzinger F, Qi Y, Meghji Z, Lou W, et al. The effect of montelukast, budesonide alone, and in combination on exercise induced bronchoconstriction. J Allergy Clin Immunol 2012;130:535-9. doi: 10.1016/j.jaci.2012.02.051 [Crossref]
- Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol 2015;136(3):556-68. doi: 10.1016/j.jaci.2015.04.047 [Crossref]
- Christensen P, Thomsen SF, Rasmussen N, Backer V, et
al. Exercise induced laryngeal obstructions objectively assessed using EILOMEA.
Eur Arch Otorhinolaryngol 2010;267:401-7. doi: 10.1007/s00405-009-1113-6 [Crossref]
- Nielsen EW, Hull JH, Backer V. High prevalence of exercise-induced laryngeal obstruction in athletes. Med Sci Sports Exerc 2013;45(11):2030–5. doi: 10.1249/MSS.0b013e318298b19a [Crossref]
- Rundell KW,
Wilber RL, Szmedra L, Jenkinson DM, Mayers LB, Im J. Exercise induced
asthma screening of elite athletes: field versus laboratory exercise challenge.
Med Sci Sports Exerc 2000;32:309-16. doi: 10.1097/00005768-200002000-00010
- Lakin RC, Metzger WJ, Haughey BH. Upper airway obstruction presenting as exercise-induced asthma. Chest 1984;86(3):499–501. doi: 10.1378/chest.86.3.499 [Crossref]
- Johansson H, Norlander K, Berglund L, Janson C, Malinovschi A, Nordvall L, Nordang L, et al. Prevalence of exercise-induced bronchoconstriction and exercise-induced laryngeal obstruction in a general adolescent population. Thorax 2015;70(1):57–63. doi: 10.1136/thoraxjnl-2014-205738 [Crossref]
- Zealear DL, Billante CR. Neurophysiology of vocal fold paralysis. Otolaryngol Clin North Am 2004;37(1):1–23. doi: 10.1016/S0030-6665(03)00165-8 [Crossref]
- Halvorsen T, Walsted ES, Bucca C, Bucca C, Bush A, Cantarella G, et al. Inducible laryngeal obstruction (ILO) - an official joint European Respiratory Society and European Laryngological Society statement. Eur Respir J 2017;50. doi: 10.1183/13993003.02221-2016 [Crossref]
- Hull JH, Backer V, Gibson PG, Fowler SJ. Laryngeal
dysfunction - assessment and management for the clinician. Am J Respir Crit
Care Med 2016;194(9):1062-72. doi: 10.1164/rccm.201606-1249C [Crossref]I
- Lakin RC, Metzger WJ, Haughey
BH. Upper airway obstruction presenting as exercise-induced asthma. Chest
1984;86(3):499-501. doi: 10.1378/chest.86.3.499 [Crossref]
- Morris MJ, Deal LE, Bean DR, Grbach VX, Morgan JA. Vocal cord dysfunction in patients with exertional dyspnea. Chest 1999;116(6):1676-82. doi: 10.1378/chest.116.6.1676 [Crossref]
- Ben-Shoshan M, Clarke AE. Anaphylaxis: past, present and future. Allergy 2011;66(1):1-14. doi: 10.1111/j.1398-9995.2010.02422.x [Crossref]
- Kim H, Fischer D. Anaphylaxis. Allergy, Asthma Clin
Immunol 2011;7(Suppl 1):1-7.
- Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69(8):1026-45. doi: 10.1111/all.12437 [Crossref]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudoallergic drug reactions. Nature. 2015;519(7542):237-41. doi: 10.1038/nature14022 [Crossref]
- Gonçalves DG, Giavina-Bianchi P. Receptor MrgprX2 nas anafilaxias não alérgicas. Arq Asma Alerg Imunol 2018;2(4). doi: 10.5935/2526-5393.20180056 [Crossref]
- Guia
para manejo da anafilaxia-2012 – Grupo de Anafilaxia da ASBAI. Rev
Bras Alerg Imunopatol
2012;35(2).
- Ben-Shoshan M, Clarke AE. Anaphylaxis: past, present and future. Allergy 2011;66(1):1-14. doi: 10.1111/j.1398-9995.2010.02422.x [Crossref]
- Maulitz RM, Pratt DS, Schocket AL. Exercise-induced anaphylactic reaction to shellfish. J Allergy Clin Immunol 1979;63(6):433-4. doi: 10.1016/0091-6749(79)90218-5 [Crossref]
- Miller CWT, Guha B, Krishnaswamy G. Exercise-induced anaphylaxis: a serious but preventable disorder. Phys Sportsmed 2008;36(1):87-94. doi: 10.3810/psm.2008.12.16 [Crossref]
- Shadick NA, Liang MH, Partridge AJ, Bingham C, Wright E, Fossel AH, et al. The natural history of exercise-induced anaphylaxis: Survey results from a 10-year follow-up study. J Allergy Clin Immunol. 1999;104(1):123-7. doi: 10.1016/s0091-6749(99)70123-5 [Crossref]
- Wong GK, Krishna MT. Food-dependent exercise-induced anaphylaxis: Is wheat unique? Curr Allergy Asthma Rep 2013;13(6):639-44. doi: 10.1007/s11882-013-0388-2 [Crossref]
- Bianchi A, Di Rienzo Businco A, Bondanini F, Mistrello G, Carlucci A, Tripodi
S. Rosaceae-associated exercise-induced anaphylaxis with positive SPT and
negative IgE reactivity to Pru
p 3. Eur Ann Allergy Clin Immunol 2011;43(4):122-4.
- Matsuo H, Morimoto K, Akaki T, Kaneko S, Kusatake K, Kuroda T, et al. Exercise and aspirin increase levels of circulating gliadin peptides in patients with wheat-dependent exercise induced anaphylaxis. Clin Exp Allergy 2005;461-6. doi: 10.1111/j.1365-2222.2005.02213.x [Crossref]
- Geller M. Anafilaxia induzida por exercício. Braz J Allergy Immunol 2015;3(2). doi: 10.5935/2318-5015.20150010 [Crossref]
- Giannetti MP.
Exercise-induced anaphylaxis: literature review and recent updates. Curr Allergy Asthma Rep 2018;18(12):72. doi: 10.1007/s11882-018-0830-6 [Crossref]
- Asaumi T, Yanagida N, Sato S, Shukuya A, Nishino M, Ebisawa M. Provocation tests for the diagnosis of food-dependent exercise-induced anaphylaxis. Pediatr Allergy Immunol 2016;27(1):44-9. doi: 10.1111/pai.12489 [Crossref]
- Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018;73:1393-414. doi: 10.1111/all.13397 [Crossref]
- Schwartz LB, Delgado L, Craig T, Bonini S, Carlsen KH, Casale TB, et al. Exercise-induced hypersensitivity syndromes in recreational and competitive athletes: a PRACTALL consensus report (what the general practitioner should know about sports and allergy). Allergy 2008;63(8):953-61. doi: 10.1111/j.1398-9995.2008.01802.x [Crossref]
- Dortas Junior SD, Azizi GG, Sousa ACMCFF, Lupi O, França AT, Valle SOR. Urticárias crônicas induzidas: revisão do tema. Arq Asma Alerg Imunol 2020. doi: 10.5935/2526-5393.20200047 [Crossref]
- Fukunaga A, Washio K, Hatakeyama M, Oda Y, Ogura K, Horikawa T, Nishigori C. Cholinergic urticaria: epidemiology, physiopathology, new categorization, and management. Clin Auton Res 2018;28(1):103-13. doi: 10.1007/s10286-017-0418-6 [Crossref]
- Magerl M, Altrichter S, Borzova E, et al. The definition, diagnostic testing, and management of chronic inducible urticarias – TheEAACI/GA(2) LEN/EDF/UNEV consensus recommendations 2016 update and revision. Allergy 2016;71(6):780-802. doi: 10.1111/all.12884 [Crossref]
- Del Giacco SR, Carlsen KH, Du Toit G. Allergy and sports in children. Pediatr Allergy Immunol 2012;23(1):11-20. doi: 10.1111/j.1399-3038.2011.01256.x [Crossref]
- Dortas Junior S, Azizi G, Valle S. Efficacy of omalizumab in chronic spontaneous urticaria associated with chronic inducible urticaria. Ann Allergy Asthma Immunol 2020;125(4):486-7. doi: 10.1016/j.anai.2020.06.011 [Crossref]
- Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med 2020;382(12):1136-48. doi: 10.1056/NEJMra1808012 [Crossref]
- Giavina-Bianchi P, Arruda LK, Aun MV, Campos RA, Chong-Neto HJ, Constantino-Silva RN, et al. Diretrizes brasileiras para o diagnóstico e tratamento do angioedema hereditário - 2017. Arq Asma Alerg Imunol 2017;1(1):23-48. doi: 10.5935/2526-5393.20170005 [Crossref]
- Ariano A, D'Apolito M, Bova M, Bellanti F, Loffredo S, D'Andrea G, et al. A myoferlin gain-of-function variant associates with a new type of hereditary angioedema. Allergy 2020;75(11):2989-92. doi: 10.1111/all.14454 [Crossref]
- Ashrafian H. Hereditary angioedema in a martial arts family. Clin J Sport Med 2005;15(4):277-8. doi: 10.1097/01.jsm.0000171884.12174.6a [Crossref]
- Valle SOR, Alonso MLO, Tortora RP, Abe AT, Levy SAP, Dortas SD Jr. Hereditary angioedema: Screening of first-degree blood relatives and earlier diagnosis. Allergy Asthma Proc 2019;40(4):279-281. doi: 10.2500/aap.2019.40.4213 [Crossref]
- Maurer M, Magerl M, Ansotegui I, Aygören-Pürsün E, Betschel S, Bork K, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-The 2017 revision and update. Allergy 2018;73(8):1575-96. doi: 10.1111/all.13384 [Crossref]