Rev Bras Fisiol Exerc 2021;22(5):552-61
doi: 10.33233/rbfex.v20i5.4748
ORIGINAL ARTICLE
Near-infrared spectroscopy during low-intensity blood
flow restriction resistance exercise
Espectroscopia
no infravermelho próximo durante exercício contrarresistência
de baixa intensidade com restrição de fluxo sanguíneo
Claudia
Mello Meirelles1, Cláudio de Souza Aguiar Junior1, Paulo
Sergio Chagas Gomes2
1Escola de Educação Física do Exército,
Rio de Janeiro, RJ, Brazil
2Instituto de Educação Física e
Desportos, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Received:
May 3, 2021; Accepted: Aug
25, 2021.
Correspondence: Paulo Sergio Chagas Gomes, Instituto de Educação Física e
Desportos, Universidade do Estado do Rio de Janeiro, Rua São Francisco Xavier,
524, 8º Andar Bloco F Sala 8104 20550-900 Rio de Janeiro, RJ, Brazil
Cláudia de Mello Meirelles: claudiameirelles@yahoo.com.br
Cláudio de Souza Aguiar Junior: sgtcpclaudio@hotmail.com
Paulo Sergio Chagas Gomes: gomespscg@yahoo.com.br
Abstract
Introduction: Low-intensity
resistance exercises with blood flow restriction are known to be effective in
promoting muscular strength and hypertrophy; however, there is a paucity of
evidence on their acute hemodynamic responses. Objective: To compare the
changes in muscular oxyhemoglobin (O2Hb), deoxyhemoglobin (HHb) concentrations, and O2 saturation (StO2)
during low load exercise under free blood flow (FreeBF)
and blood flow restriction (BFR). Methods: Fifteen healthy males were
subjected to bilateral knee extension tests under FreeBF
and BFR conditions, in a random order. The knee extension exercise included
four sets of 15 repetitions at 20% of one-repetition maximum, with 30s interval
between the sets. In the BFR condition, subjects exercised with a cuff
positioned on the proximal thigh and inflated to 50% of total occlusion
pressure. Changes in the O2Hb, HHb, total
hemoglobin (tHb), and StO2 in vastus
lateralis muscle were monitored using near-infrared spectroscopy. Results:
A two-way repeated-measures ANOVA revealed significant main effects for sets
for all variables (P < 0.05). Moreover, the values in StO2 during
sets 2, 3, and 4 in BFR conditions were significantly lower than those in freeBF. Significant differences were also seen between the
exercise conditions during rest intervals for HHb
(rest intervals 2, 3, and 4) and tHb (rest interval
3; P < 0.05). There were no significant interactions between conditions and
sets or conditions and intervals for O2Hb. Conclusion:
Low-intensity resistance exercise performed with BFR significantly decreased
the acute muscle StO2 and increased total muscle hemoglobin.
Keywords: resistance training; muscle,
skeletal; muscle strength; near-infrared spectroscopy.
Resumo
Introdução: Exercícios contrarresistência
de baixa intensidade com restrição do fluxo sanguíneo (RFS) são conhecidos por
serem eficazes na promoção de força e hipertrofia muscular. No entanto, há uma
escassez de evidências sobre suas respostas hemodinâmicas agudas. Objetivo:
Comparar as alterações nas concentrações musculares de oxihemoglobina
(O2Hb), desoxihemoglobina (HHb) e saturação de O2 (StO2) durante
exercício contrarresistência com baixa intensidade
com fluxo sanguíneo livre (FSL) e RFS. Métodos: Quinze homens saudáveis
foram submetidos aleatoriamente a testes de extensão bilateral do joelho (4
séries/15 repetições a 20% de 1RM, intervalo de 30s entre séries) nas condições
FSL e RFS. Na condição RFS, os voluntários se exercitaram com um manguito
posicionado na região proximal da coxa e inflado a 50% da pressão de oclusão.
Alterações no O2Hb, HHb, hemoglobina total
(tHb) e StO2 no músculo vasto lateral
foram monitorados usando espectroscopia no infravermelho próximo. Resultados:
Uma ANOVA de duas vias com medidas repetidas revelou efeitos principais
significativos em séries para todas as variáveis. Os valores de StO2 durante as
séries 2, 3 e 4 em condições de RFS foram significativamente menores do que em
FSL. Também foram observadas diferenças entre as condições de exercício durante
os intervalos de descanso para HHb (intervalos 2, 3 e
4) e tHb (intervalo 3) (todas as diferenças para P <
0,05). Não houve interações significativas entre as condições e séries ou
condições e intervalos para O2Hb. Conclusão: O exercício contrarresistência de baixa intensidade realizado com RFS
diminuiu significativamente a StO2 muscular e aumentou a hemoglobina
muscular total.
Palavras-chave: treinamento de força; músculo
esquelético; força muscular; espectroscopia de luz próxima ao infravermelho.
Introduction
Resistance
exercise with blood flow restriction (BFR-RE) has gained greater attention in
recent years since it has shown significant gains in muscle strength and
hypertrophy in athletes [1] and non-athletes [2].
Blood flow is generally
restricted by applying inflatable cuffs in the proximal region of limbs. Blood
flow restriction pressure is commonly used with 50% to 80% of the required
pressure for total occlusion of the venous blood flow, yet low enough to
maintain arterial inflow into the muscle. When combined with restricted blood
flow, resistance training is performed with 20% to 50% of an individual's
maximum repetition (1 RM) [3].
Available
evidence advocates the feasibility and safety of BFR-RE [4]; however, the underlying
mechanisms responsible for the observed results remain unclear. Potential
proposed mechanisms are still under scrutiny, but researchers have suggested
that muscle growth stimulated by BFR-RE might be related to the induced
increase in post-exercise hyperemia and cell swelling, as well as the greater
metabolic stress associated to the relative hypoxia caused by blood flow
restriction [5].
Monitoring of
the acute muscle hemodynamic responses during and after the BFR-RE might be an
opportunity to shed some light on understanding the mechanisms involved in
BFR-RE-induced hypertrophy, particularly those related to the modifications of
muscle blood volume and oxygenation. Near infrared spectroscopy (NIRS) is
widely used in the research for acute and chronic effects of resistance
exercise under various conditions [6,7,8,9,10].
To the best of
our knowledge, changes in the muscle oxygen saturation during BFR resistance
exercise remained less explored. Previous studies have adopted protocols
involving only one set [11], sets to volitional fatigue [12] or isokinetic
equipment [13,14], which limits the extrapolation of their findings to the
real world.
As the knowledge
of hemodynamic responses can be useful in understanding the phenomena behind
the BFR-RE effects, this study aimed to compare the changes in muscular
oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb)
and O2 saturation (StO2) during low-load exercise under
BFR and freeBF.
Methods
Subjects
Fifteen healthy
males (age: 18.7 ± 0.5 years; body mass: 66.8 ± 8 kg; height: 172 ± 9 cm; body
fat: 9.4 ± 4.6%) who were inexperienced in resistance training, were included
in this study. The criterion for exclusion included current musculoskeletal
injuries of lower limbs. The subjects were advised to maintain their physical
activity throughout the study period. All participants received a detailed
verbal explanation of the study procedures and risks involved in the
experimental procedures, and they signed a written informed consent form prior
to participation in the study. The study was approved by the Naval Hospital Marcílio Dias Ethical Review Board (#718.602), with the
procedures following the principles of the Declaration of Helsinki.
Study design
The study
comprised three testing sessions, with an interval of one week. The first
session determined the demographic and anthropometric data, maximal dynamic
strength, and occlusion pressure. The subject's familiarization with the
exercise protocol using the inflated cuff was also part of the first visit.
The second and
third sessions comprised bilateral knee extension tests under freeBF and BFR conditions in random order. In BFR, two
sphygmomanometers were fitted individually to both thighs. The subjects had
muscle hemodynamics changes monitored in the right limb before, during, and
after exercises in both experimental conditions.
Determination of occlusion pressure
Blood flow
occlusion pressure was determined by ultrasound equipment with Doppler (GE LogiqE, GE Healthcare, EUA). Subjects were instructed to
lie in a prone position while the cuff positioned in the proximal thigh was
inflated up to no pulse, which was detected with the Doppler probe positioned
over the popliteal artery. The pressure employed during the exercise visits was
equal to 50% of the total arterial occlusion pressure.
Resistance exercise testing
All subjects
were submitted to bilateral knee extension test using isotonic equipment
(Technogym, Rehabilitation Device, Gambettola,
Italy).
To minimize any
extraneous movement during the strength tests, participants were strapped over
the hips for immobilization of the hip joint. All individual settings
accomplished during that visit were recorded and replicated in the subsequent
experiment.
Maximum dynamic
strength was determined with the one-repetition maximal test (1 RM) in the same
isotonic equipment using in the experimental visits. The protocol comprised ten
submaximal repetition warm-ups followed by the 1 RM test attempts (maximum 6).
Loads were added progressively, with a minimum of 3-min rest period between the
attempts. The highest load successfully lifted was recorded as the subject’s 1
RM.
Experimental
tests comprised four sets of 15 repetitions at 20% 1 RM, with 30 s intervals
between sets. Only repetitions executed over the complete range of 90° motion
were considered valid. Subjects performed the movement at their convenience
while receiving verbal stimulus from the testers towards the end of the
pre-determined exercise. For BFR sessions, a commercial nylon-cuffed
thigh-sized aneroid sphygmomanometer (70 cm x 16 cm tourniquet) (B.Well Swiss AG, Switzerland) was
placed around the proximal portion of both thighs individually. Inflation pressure
applied was equivalent to 50% of the arterial occlusion pressure determined
previously. Inflation started just before the exercise began and was released
shortly after the last repetition.
Muscle hemodynamics monitoring with NIRS
Muscle
hemodynamics were monitored continuously in real-time by near-infrared
spectroscopy (Oxiplex TS, ISS, Champaign, IL, USA).
Total muscle microvascular concentrations of hemoglobin and myoglobin was
measured for 1-min rest during the strength test, and the 30-s rest period. The
instrument uses near-infrared light at two wavelengths (690 and 830 nm) with an
optical fiber-based light and detector source, providing absolute values of
tissue hemoglobin saturation, and individual concentration of O2Hb
and HHb. Throughout the entire exercise protocol tHb (sum of O2Hb and HHb)
and in StO2 were monitored in real-time.
The NIRS probe
was initially covered with a plastic film to avoid humidity from the skin. The
probe was placed on the skin over the vastus lateralis muscle of the subject’s
right thigh, while seated, 2 to 3 cm from the outer limit of the cuff. To
secure it on the skin and minimize movement during exercise, an elastic bandage
was wrapped around the subject’s thigh and covered with a black towel, reducing
the possibility of extraneous light influencing the signal. The adipose
thickness at the subject's thigh did not impair light penetration because the
measured thickness (Harpenden Skinfold Caliper, Baty International, West Sussex, United Kingdom) was less
than 25 mm, the depth of penetration of the NIRS. The NIRS device was
calibrated before every test as recommended by the manufacturer. All data were
collected online at a frequency of 1 Hz, using specific dedicated software.
The NIRS data were
recorded throughout the exercise period, including intervals between sets. For
analysis purposes, the lowest values of tHb, O2Hb,
and StO2 were recorded, as well as the highest values of HHb obtained during the series. During the rest intervals,
the highest values of tHb, O2Hb, and StO2,
and the lowest values of HHb were also recorded, as
well as the last 10 s of baseline.
Unpublished data
from our laboratory have demonstrated the test-retest intraclass correlation
coefficients for muscle oxygenation (minimum or amplitude values, either on the
same day or on two separate testing days), ranging from R = 0.724 to 0.989.
Statistical analysis
All data are
presented as mean and standard deviation. Two-way repeated measures ANOVA was
used to identify the differences in NIRS variables, obtained during the
exercise stages (sets and rest intervals separately) between FreeBF and BFR conditions. All statistical analyses were
performed using a commercially available software (SPSS for Mac, Ver. 20.0,
Armonk, NY: IBM Corp). All statistical analyses were tested at 95% probability.
Results
None of the
subjects reported any side effects during or after the exercise protocols. One
repetition maximum for the bilateral leg extension exercise was 122 ± 17 kg,
and the load applied in tests was 24 ± 3 kg (20% 1 RM). The mean inflation cuff
pressure for total popliteal artery occlusion was 168 ± 19 mmHg, and the
inflation pressure applied during BFR exercise condition was 84 ± 10 mmHg (50%
total occlusion). The O2Hb, HHb, StO2,
and tHb trends in a typical subject on freeBF and BFR conditions are illustrated in Figure 1.
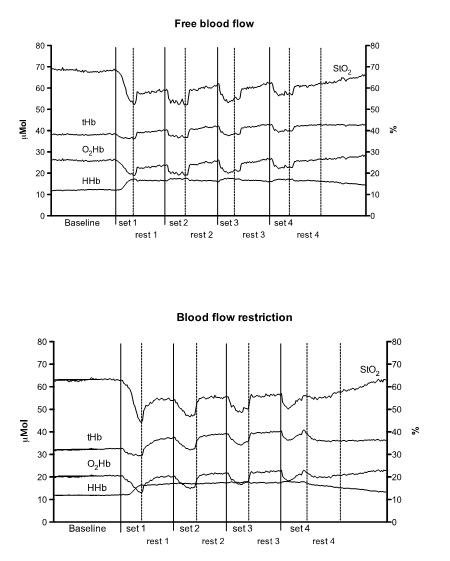
Figure 1 - Muscle
NIRS variables trend during four sets of knee extension in free blood flow
(upper panel) and blood flow restriction (lower panel) conditions
Figure 2
presents the mean values of NIRS variables in each exercise condition and
stage. For all NIRS variables there were significant main effects of sets.
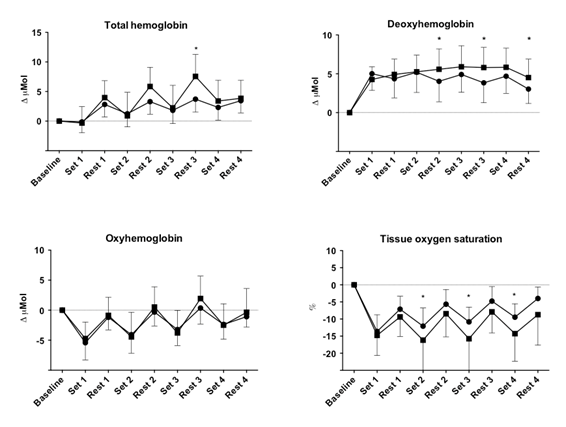
Asterisks indicate
significant interactions between conditions
Figure 2 - Mean
changes in NIRS variables during four sets of knee extension in free blood flow
(circles) and blood flow restriction (squares) conditions
No statistically
significant interactions (condition × sets or condition × rest intervals) were
observed for O2Hb. Significant lower values were observed for StO2
during sets 2, 3, and 4 in BFR condition as compared to those in freeBF. Moreover, significant differences were seen between
the exercise conditions during rest intervals for HHb
(rest intervals 2, 3, and 4) and tHb (rest interval
3).
Discussion
This study
assessed the NIRS variable responses with and without BFR to understand the
effects of blood restriction on muscle O2Hb and HHb
concentrations and StO2 during knee extension. Our results
contributed mainly to a better understanding of the hemodynamic responses
during resistance exercise performed in isotonic equipment, comprising a
predetermined repetition count instead of a fatigue protocol. Another important
aspect is the use of a restriction pressure related to the individual's
estimated arterial occlusion, strengthening the external validity of the
results. Besides, the application of 50% arterial occlusion was appropriate to
ensure comfort to the participant with accurate blood flow restriction, as
reported in a study by Mouser et al. [15], wherein the blood flow was
relatively unchanged between 50% and 90% of arterial occlusion pressure.
Furthermore, low to moderate relative pressure (40% to 50% estimated arterial
occlusion) is sufficient to maximize the acute response to BFR exercise.
The NIRS data
collected during the knee extension exercise revealed that from the third
resting exercise interval, muscle tHb was higher for
the BFR condition. A significant increase was observed in HHb
concentration in the BFR compared to FreeBF during
rest intervals. Traditionally, this represents an increase in oxygen
consumption by the muscle [16]. Nevertheless, the overall findings (similar O2Hb,
higher tHb, and lower StO2 in BFR
condition) revealed that presumably, the venous pooling during the rest
intervals led to this event, rather than an actual increase in the muscular
oxygen consumption.
Venous blood
accumulation can favor blood influx in the sarcoplasm, causing cell swelling
and accumulation of metabolites. One of the possible components explaining
muscle hypertrophy is acute cell edema since it can stimulate protein synthesis
and suppress proteolysis [17]. In this study, possibly the higher muscle tHb observed during the knee extensions could have helped
augment the intracellular swelling due to osmotic water shifts into the cell,
thus collaborating to the understanding of the hypertrophic effect of
low-intensity resistance exercises with BFR. This issue needs to be
investigated further to confirm this hypothesis.
Yasuda et al.
[18] found that immediately after performing 75 repetitions of concentric
biceps curls at 30% 1 RM, the elbow flexor muscle thickness acutely increased
by 11.7%. Following a six-week training period, it was observed a significant
12% increase in MRI-measured muscle cross-sectional area of the elbow flexors.
Authors suggested that the pronounced muscle cell swelling might crucially
promote muscle hypertrophy.
Although no
between-condition differences were observed in O2Hb, since the
applied blood flow restriction did not limit the arterial influx, a significant
lower muscle StO2 was observed from set 2 to set 4 performed under
BFR, representing the hypoxic characteristic of BFR exercise. These results
were consistent with those by previous studies adopting the exercise protocols
involving repetitions to volitional fatigue [12] during isotonic [11,14] and
isokinetic movement [13].
In accordance to Loenneke et al. [19],
hypoxia can be adequate for enhancing the recruitment of high-threshold motor
units, as well as provoking systemic elevation of hormonal and growth factors
[20] and activation and proliferation of myogenic stem cells [3], thus
promoting the hypertrophic response to perform the exercise.
During BFR
exercise, due to the hypoxia conditions, seems to occur additional recruitment
of more motor units to compensate for the oxygen deficit. Training can recruit
more fast-twitch fibers than traditional resistance exercise does [21].
Another possible
beneficial effect explaining the hypertrophic mechanisms unleashed by BFR is a
higher anabolic hormonal response even with a lower training load, probably
entailed by its inherent muscular hypoxia. Manini et al. [22] reported
that low-load BFR resistance exercise was able to stimulate growth hormone (GH)
secretion in an amount comparable to that produced by high-load resistance
exercise without BFR.
As reported by
Nielsen et al. [3] the exact mechanism underlying this phenomenon is not
known in respect to increasing in myogenic stem cell proliferation. However,
the authors believe that BFR-induced hypoxia and/or nitric oxide production may
stimulate this process, which is required to cause substantial increases in
muscle fiber cross-sectional area [23].
It could also be
reasonable to speculate that low oxygen tension is one of the physiological
signals related to the capacity of resistance exercise in inducing
angiogenesis. Notably, during resistance exercise with BFR, the decrease in
oxygen muscle saturation might stabilize hypoxia-inducible factor 1 alfa (HIF-1
alfa), activating vascular endothelial growth factor (VEGF) transcription, the
main vascular growth factor. This cascade in angiogenesis would be involved in
facilitating metabolic improvements in muscle cells helping in protein
synthesis [24,25].
A limitation of
the present study was the application of cuff occlusion immediately before the
exercise commencement, which may have blunted the observed results. Cayot et al. [21] revealed that the occlusion
pressure applied 5 min before isometric knee extension exercise elicited
greater changes in HHb when compared to the
immediately occluding pressure before exercise, suggesting that a higher
occlusion time may be necessary to amplify the BFR-induced metabolic stress.
Conclusion
In conclusion,
our findings indicate that exercise performed with blood flow restriction,
based on 50% of total blood occlusion, promoted significant decreases in the
acute muscle StO2 and an increase in muscle tHb
during the exercise. As previously observed, significantly lower levels in StO2
represent greater metabolic stress when associated with the relative hypoxia
caused by blood flow restriction. In contrast, the higher tHb
at the end of each set suggests that the muscle growth stimulated by BFR-RE
might be related to their induced increase in post-exercise hyperemia and cell
swelling.
Further studies
are warranted to monitor the influx of arterial blood flow during various
degrees of occlusion, as well as to investigate the variables involved in the
high individual variations in hemodynamic responses during the resistance
exercise under blood flow restriction.
Potential conflict of
interest
No potential conflicts
of interest relevant to this article have been reported
Financing source
No external financing
was used in this study
Author’s contributions
Research conception and
design: Meirelles CM; Data collection: Aguiar Jr, CS and Meirelles CM; Analysis
and interpretation of data: Meirelles CM, Aguiar Jr, CS and Gomes PSC;
Statistical analysis: Meirelles CM and Gomes PSC; Writing of the manuscript:
Meirelles CM and Gomes PSC; Critical review of the manuscript for important
intellectual content: Meirelles CM and Gomes PSC. All the authors have
contributed substantially to the manuscript and approved the final submission
References
- Scott BR, Loenneke JP,
Slattery KM, Dascombe BJ. Blood flow restricted
exercise for athletes: A review of available evidence. J Sci Med Sport 2016;19:360-7. doi: 10.1016/j.jsams.2015.04.014 [Crossref]
- Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Exercise with blood flow restriction: an updatred evidence-based approach for enhanced muscular development. Sports Med 2015;45:313-25. doi: 10.1007/s40279-014-0288-1 [Crossref]
- Nielsen JL, Aagaard P, Bech RD, Tobias N, Lars Grøndahl H, Mathias W, et al. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol 2012;590(Pt 17):4351-61. doi: 10.1113/jphysiol.2012.237008 [Crossref]
- Loenneke JP, Wilson JM, Wilson GJ, Pujol TJ, Bembem MG. Potential safety issues with blood flow restriction training. Scand J Med Sci Sports 2011;21:510–8. doi: 10.1111/j.1600-0838.2010.01290.x [Crossref]
- Suga T, Okita K, Takada S, Omokawa M, Kadoguchi T, Yokota T, et al. Effect of multiple set on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. Eur J Appl Physiol 2012;112:3915-20. doi: 10.1007/s00421-012-2377-x [Crossref]
- Gomes OS, Matsuura C, Bhambhani YN. Effects of hypoxia on cerebral and muscle haemodynamics during knee extensions in healthy subjects. Eur J Appl Physiol 2013;113:13-23. doi: 10.1007/s00421-012-2408-7 [Crossref]
- Alvares TS, Conte Junior CA, Paschoalin VM, Silva JT, Meirelles CM, Bhambhani YN, et al. Acute L-arginine supplementation increases muscle blood volume but not strength performance. Appl Physiol Nutr Metab 2012;37:115-26. doi: 10.1139/h11-144 [Crossref]
- Matsuura C, Gomes PS, Haykowsky M, Bhambhani YN. Cerebral and muscle oxygenation changes during static and dynamic knee extensions to voluntary fatigue in healthy men and women: a near infrared spectroscopy study. Clin Physiol Funct Imaging 2011;31:114-23. doi: 10.1111/j.1475-097X.2010.00986.x [Crossref]
- Pereira MI, Gomes PS, Bhambhani YN. Acute effects of sustained isometric knee extension on cerebral and muscle oxygenation responses. Clin Physiol Funct Imaging 2009;29:300-8. doi: 10.1111/j.1475-097X.2009.00870.x [Crossref]
- Pereira MIR, Gomes PSC, Bhambhani YN. A Brief Review of the use of near infrared spectroscopy with particular interest in resistance exercise. Sports Med 2007;37:615-24. doi: 10.2165/00007256-200737070-00005 [Crossref]
- Singer TJ, Stavres J, Elmer SJ, Kilgas MA, Pollock BS, Kearney SG, et al. Knee extension with blood flow restriction: Impact of cuff pressure on hemodynamics. Eur J Appli Physiol 2020;120(1):79-90. doi: 10.1007/s00421-019-04250-2 [Crossref]
- Downs ME, Hackney KJ, Martin D, Caine TL, Cunningham D, O'Connor DP, et al. Acute vascular and cardiovascular responses to blood flow-restricted exercise. Med Sci Sports Exerc 2014;46:1489-97. doi: 10.1249/MSS.0000000000000253 [Crossref]
- Ganesan, Cotter JA, Reuland W, Cerussi AE, Tromberg BJ, Galassetti P. Effect of blood flow restriction on tissue oxygenation during knee extension. Med Sci Sports Exerc 2015;47:185-93. doi: 10.1249/MSS.0000000000000393 [Crossref]
- Reis JF, Fatela P, Mendonca GV, Vaz JR, Valamatos MJ, Infante J, et al. tissue oxygenation in response to different relative levels of blood-flow restricted exercise. Front Physiol 2019;10:407. doi: 10.3389/fphys.2019.00407 [Crossref]
- Mouser JG, Dankel SJ, Jessee MB, Mattocks KT, Buckner SL, Counts BR, et cols. A tale of three cuffs: the hemodynamics of blood flow restriction. Eur J Appl Physiol 2017;117(7):1493-9. doi: 10.1007/s00421-017-3644-7 [Crossref]
- Ferreira LF, Koga S, Barstow TJ. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J Appl Physiol 2007;103:1999-2004. doi: 10.1152/japplphysiol.01414.2006 [Crossref]
- Lang F, Busch GL, Ritter M, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 1998;78:247-306. doi: 10.1152/physrev.1998.78.1.247 [Crossref]
- Yasuda T, Loenneke JP, Thiebaud RS, Abe T. Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS One 2012;7:e52843. doi: 10.1371/journal.pone.0052843 [Crossref]
- Loenneke JP, Kim D, Fahs CA, Thiebaud RS, Abe T, Larson RD, et cols. Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle Nerve 2015;51:713-21. doi: 10.1002/mus.24448 [Crossref]
- Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, et al. Mechanical stimuli regulate rapamycin-sensitive signalling by a phospho-inositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J 2004;380(Pt3):795-804. doi: 10.1042/BJ20040274 [Crossref]
- Hwang PS, Willoughby DS. Mechanisms behind blood flow-restricted training and its effect toward muscle growth. J Strength Cond Res 2019;Suppl 1:S167-79. doi: 10.1519/JSC.0000000000002384 [Crossref]
- Manini TM , Yarrow JF, Buford
TW, Clark BC, Conover CF, Borst SE. Growth hormone responses to acute
resistance exercise with vascular restriction in young and old men. Growth Horm IGF Res 2012;22(5):167-72. doi: 10.1016/j.ghir.2012.05.002 [Crossref]
- Loenneke JP, Thiebaud RS, Fahs CA, Rossow LM.
Blood flow-restricted resistance exercise: rapidly affecting the myofibre and the myonuclei. J Physiol
2012;590(Pt 21):5271. doi: 10.1113/jphysiol.2012.242859 [Crossref]
- Ferguson RA, Hunt JE, Lewis MP, Martin NR, Player DJ, Stangier C, et al. The acute angiogenic signaling response to low load resistance exercise with blood flow restriction. Eur J Sport Sci 2018; 18(3):397-406. doi: 10.1080/17461391.2017.1422281 [Crossref]
- Cayot TE, Lauver JD, Silette CR, Scheuermann BW. Effects
of blood flow restriction duration on muscle activation and microvascular
oxygenation during low-volume isometric exercise. Clin Physiol Funct Imaging 2016;36:298-305. doi: 10.1111/cpf.12228 [Crossref]