Rev Bras Fisiol Exerc 2021;20(4):490-502
doi: 10.33233/rbfex.v20i4.4752
REVIEW
Biomarkers of tissue injury in high-intensity interval
running: a systematic review
Biomarcadores
de lesão tecidual em corrida intervalada de alta intensidade: uma revisão
sistemática
Thiago
Dias Sales1,2, Danielli Braga de Mello3, Wagner Siqueira
Romão1,3, Rodolfo de Alkmim Moreira Nunes1,
Eduardo Borba Neves2, Juliana Brandão Pinto de Castro1,
Rodrigo Gomes de Souza Vale1,4
1Universidade do Estado do Rio de
Janeiro, Rio de Janeiro, RJ, Brasil
2Comissão de Desportos do Exército, Rio
de Janeiro, RJ, Brasil
3Escola de Educação Física do Exército,
RJ, Brasil
4Universidade Estácio de Sá, Cabo Frio,
RJ, Brasil
Received:
May 4, 2021; Accepted: July 12, 2021.
Correspondence: Thiago Dias Sales, Comissão de Desportos do Exército,
Almirante Floriano Peixoto, s/n Urca 22291-090 Rio de Janeiro RJ
Thiago Dias Sales: thiago_tds90@yahoo.com.br
Danielli Braga de Mello: danielli.mello@gmail.com
Wagner Siqueira Romão: capromao.cde@gmail.com
Rodolfo de Alkmim Moreira Nunes:
rodolfoalkmim@gmail.com
Eduardo Borba Neves: borbaneves@hotmail.com
Juliana Brandão Pinto de Castro:
julianabrandaoflp@hotmail.com
Rodrigo Gomes de Souza Vale: rodrigovale@globo.com
Abstract
Introduction: The
improvement of aerobic and anaerobic capacity in athletes of different sports
is related to high-intensity exercise performance, which causes cellular
microlesions and leads to an inflammatory process necessary for muscle
adaptation. Biochemical markers, such as creatine kinase (CK) and lactate
dehydrogenase (LDH), have been used to measure muscle and inflammatory damage
to identify the physiological response and improving sports performance. Objective:
To describe the changes in the CK and LDH biomarkers after interval running at
high intensity. Methods: We conducted a systematic review following the
PRISMA guidelines and registered on PROSPERO (CRD42020201678), with a
literature search, in February 2021, in the Medline, Lilacs, Scopus, SPORTDiscus, CINAHL, Web of Science, ScienceDirect,
Cochrane, and Scielo databases. We used the descriptors
“HIIT”, “L-Lactate Dehydrogenase”, “Creatine Kinase” and their synonyms,
available in the Health Sciences Descriptors (DeCS)
and Medical Subject Headings (MeSH). Results:
From the 80 studies found, 6 met the inclusion criteria. Of these, four studies
showed significant increases in CK and LDH simultaneously, while one study
observed a significant increase only in CK and the other study only in LDH. The
increases in biomarkers occurred at different magnitudes. The studies’
protocols and the sample characteristics showed high heterogeneity. Conclusion:
High-intensity interval running can acutely elevate CK and LDL levels, making
them excellent markers for injury risk and exercise load dosing.
Keywords: high-intensity interval training;
creatine kinase; lactate dehydrogenase.
Resumo
Introdução: A melhora da capacidade aeróbia e
anaeróbia em atletas de diferentes modalidades esportivas está relacionada à
realização de exercícios de alta intensidade, que causam microlesões
celulares e levam a um processo inflamatório necessário para adaptação
muscular. Marcadores bioquímicos, como creatina quinase (CK) e lactato
desidrogenase (LDH) vêm sendo utilizados para a mensuração de danos musculares
e inflamatórios a fim de identificar a resposta fisiológica e auxiliar na
melhora do desempenho esportivo. Objetivo: Descrever as alterações nos
biomarcadores CK e LDH após a execução de corrida intervalada em alta
intensidade. Métodos: Foi realizada uma revisão sistemática, seguindo as
recomendações do PRISMA e registrada na PROSPERO (CRD42020201678), com uma
busca na literatura em fevereiro de 2021, nas bases Medline, Lilacs, Scopus, SPORTDiscus,
CINAHL, Web of Science, ScienceDirect,
Cochrane e Scielo, utilizando os descritores “HIIT”,
“L-Lactate Dehydrogenase”,
“Creatine Kinase” e seus
sinônimos, disponíveis nos Descritores em Ciências da Saúde (DeCS) e Medical Subject Headings (MeSH). Resultados:
Dos 80 estudos encontrados inicialmente, 6 atenderam aos critérios de inclusão.
Destes, quatro estudos apresentaram aumento significativos de CK e LDH
simultaneamente, enquanto 1 estudo observou aumento significativo apenas de CK
e o outro estudo apenas de LDH. Os aumentos nos biomarcadores ocorreram em
magnitudes diferentes. Os protocolos dos estudos e as características da amostra
mostraram alta heterogeneidade. Conclusão: A corrida intervalada de alta
intensidade pode elevar os níveis CK e LDL de forma aguda, o que torna os
mesmos excelentes marcadores para o risco de lesão e dosagem das cargas do
exercício.
Palavras-chave: treinamento intervalado de alta
intensidade; creatina quinase; lactato desidrogenase.
Introduction
High-intensity
interval training (HIIT) is a widely used and effective training method in many
sports, including endurance and sprint/power events [1]. According to different
combinations of work intensity and session length, HIIT uses different work
interval protocols, including long interval (2-4 min of work/session at
submaximal intensity, LI-HIIT), short interval (< 45 s of work/session at
submaximal intensity, SI-HIIT), sprint interval (> 20-30 s of work/session
close to maximum intensity, SIT), and repeated sprint exercises (≤ 10 s
of work/session close to maximum intensity, RST). When the number of
repetitions is increased, HIIT protocols can be implemented with high (16 min
work) or low (4 min work) session volume (HV-HIIT or LV-HIIT) [2].
HIIT requires an
integration of several physiological systems. The contributions of
ATP-phosphocreatine (PCr) and the glycolytic
metabolic pathway are necessary to achieve high exercise intensity, while an
oxidative metabolic pathway is predominant to maintain high exercise intensity as long as possible [3].
High-intensity
exercises have benefits for athletes of different modalities [4] and are
related to a series of aerobic and anaerobic adaptations, such as the increase
in the dimensions of mitochondria, greater tolerance to blood pH, and increased
anaerobic capacity [5]. However, strenuous, high-intensity exercise can have
unfavorable effects when the workload is not controlled [6], which can cause
severe damage to muscle tissue. Some enzymes are used as indicators of tissue
damage. Among these enzymes, creatine kinase (CK) and lactate dehydrogenase
(LDH) are capable of stimulating inflammation and muscle damage because of the
physical stimulus suffered by the body [7].
CK is an
intramuscular enzyme that accelerates the resynthesis of ATP
and its increases are noticed in blood dosages after strenuous activities [8].
Generally, the peak concentration occurs between 24 and 48 hours after exercise
and returns to baseline values between 48 and 120 hours, depending on the peak
magnitude [9].
LDH is an enzyme
that is in the cytoplasm of most cells and is responsible for catalyzing the
reaction that results in the conversion of pyruvate to lactate [10]. Like CK,
LDH is associated with muscle injuries [11].
The time of
detection of CK in the blood is dependent on the level of training, type,
intensity, and duration of the exercise. CK values vary widely between
individuals and may change according to sex, age, amount of muscle mass, race,
level of training, and climatic condition. Likewise, LDH has post-exercise
variations and can also change with the training level of the individual [12].
The
understanding of the dynamics of expression of these biochemical markers and
its functional criteria can help in the training load adjustments, and
thereafter to adaptations in the athletes' organism facing this type of
exercise [13]. Thus, studies that investigate the acute effects of physical
exercise on inflammatory markers, usually done with blood collection before and
immediately after physical activity, are important for the relationship between
training and performance [14].
Therefore, the
present study aimed to describe the changes in CK and LDH biomarkers after
high-intensity interval running.
Methods
Protocol and registration
This systematic
review was conducted according to the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) recommendations [15] and registered in the
International Prospective Register of Systematic Reviews (PROSPERO) as number
CRD42020201678.
Inclusion criteria
We included
studies that met the following inclusion criteria [16]: Population: running
practitioners; Exposure of interest (independent variable): high-intensity
interval running; Outcome (dependent variable): biomarkers of tissue damage CK
and LDH in individuals of both sexes. We excluded studies of systematic
reviews, meta-analyses, case studies, and studies with a publication date
before the year 2011, considering a systematic review published on this issue
in 2012 [17].
Search strategy
A systematic
literature search was conducted in February 2021, without a language filter, in
the databases National Library of Medicine (Medline), Lilacs, Scopus, SPORTDiscus, Cumulative Index to Nursing and Allied Health
Literature (CINAHL), Web of Science, ScienceDirect, Cochrane, and Scielo. We used the descriptors “HIIT”, “L-Lactate
Dehydrogenase”, “Creatine Kinase” and their synonyms, available in Health
Sciences Descriptors (DeCS) and Medical Subject
Headings (MeSH). The following search phrase was
obtained using the Boolean operators “AND” between descriptors and “OR” between
synonyms: (“High Intensity Interval Training” OR “High-Intensity Interval
Trainings” OR “Interval Training, High-Intensity” OR “Interval Trainings,
High-Intensity” OR “Training, High-Intensity Interval” OR “Trainings,
High-Intensity Interval” OR “High-Intensity Intermittent Exercise” OR
“Exercise, High-Intensity Intermittent” OR “Exercises, High-Intensity
Intermittent” OR “High-Intensity Intermittent Exercises” OR “Sprint Interval
Training” OR “Sprint Interval Trainings”) AND (“Creatine kinase” OR “Kinase,
Creatine” OR “ATP Creatine Phosphotransferase” OR “Creatine Phosphotransferase,
ATP” OR “Phosphotransferase, ATP Creatine” OR “Creatine Phosphokinase” OR
“Phosphokinase, Creatine” OR “ADP Phosphocreatine Phosphotransferase” OR
“Phosphocreatine Phosphotransferase, ADP” OR “Phosphotransferase, ADP
Phosphocreatine” OR “Macro-Creatine Kinase” OR “Macro Creatine Kinase”)
AND (“L-Lactate Dehydrogenase” OR “Dehydrogenase, L-Lactate” OR “L Lactate
Dehydrogenase” OR “Lactate Dehydrogenase” OR “Dehydrogenase, Lactate”).
Additionally,
references of the selected studies and other sources were explored to maximize
the search. Two independent evaluators selected the studies. A third researcher
resolved the disagreements between the evaluators. This procedure was performed
in all phases of the present study.
Data collection process
We extracted the
following data from the studies: profile of participants, sex, age, height,
total body mass (TBM), body fat percentage (BF%), body mass index (BMI),
maximum oxygen consumption (VO2max), assessment protocols,
biochemical analyzes of CK and LDH, and study results.
Methodological quality analysis
Methodological quality
was assessed through the Tool for the assEssment of
Study qualiTy and reporting in EXercise
(TESTEX). This tool has a 15-point scale, each item equals 1 point. The
following domains were evaluated: 1) Eligibility criteria specified; 2)
Randomization specified; 3) Allocation concealment; 4) Groups similar at
baseline; 5) Blinding of evaluator; 6) Withdrawals from the study <15%;
reported adverse events; reported session attendance; 7) Intention-to-treat
analysis; 8) Primary and secondary between-group statistical comparisons
reported; 9) Point measures for all results; 10) Activity monitoring in control
groups; 11) Relative exercise intensity remained constant; 12) Exercise energy
expenditure reported [18].
Risk of bias analysis
The A Cochrane
Risk Of Bias Assessment Tool for Non-Randomized
Studies of Interventions (ACROBAT-NRSI) was used to assess the risk of bias of
the included studies. This tool analyzes seven domains: 1) Bias due to
confounding; 2) Bias in selection of participants into the study; 3) Bias in
measurement of interventions; 4) Bias due to departures from intended
interventions; 5) Bias due to missing data; 6) Bias in measurement of outcomes;
7) Bias in selection of the reported result [19]. For each domain, the studies
were classified as uninformed, low, moderate, severe, or critical risk of bias.
For a study to be classified as “low risk”, it should be classified as low risk
in all domains. A study is classified as “critical risk” if it presents a
critical risk in at least one of the seven domains of the tool.
Results
Initially, 80
articles were identified in the searched databases (Medline = 19; Lilacs = 2;
Scopus = 0; CINAHL = 31; SPORTDiscus = 0; Web of
Science = 22; ScienceDirect = 0; Cochrane = 6; SciELO
= 0). Four studies were included manually. After using the eligibility
criteria, six studies were included in this review (Figure 1).

Figure 1 - Flow
diagram of the studies included in the systematic review
The sample
characteristics and protocols of the included studies are summarized in Table
I. The sample had a total of 84 participants, 64 male and 20 not informed.
Among the characteristics of the subjects, all six studies showed age, total
body mass, and height. Only Cipryan [20] did not
present the BMI. Three studies showed aerobic capacity (VO2max)
[20,21,22]. Only Cipryan [20] and Santos et al.
[23] presented the body fat percentage (BF%). Four studies used HIIT protocols
[19,20,21,22,24] and two used tests that resemble HIIT protocols [23,25].
Table I - Sample
characteristics and protocols
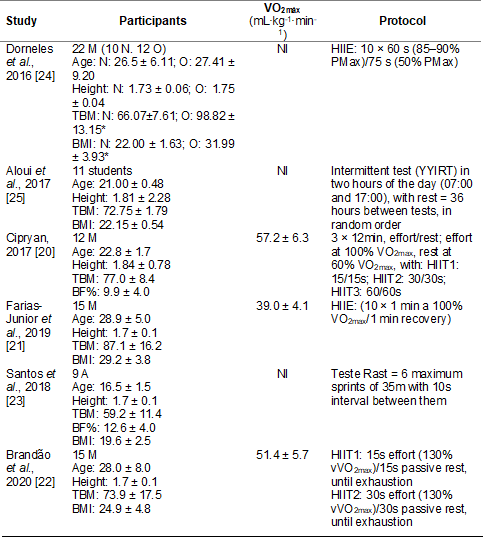
M = men; N = normal
weight; O = overweight; A = athletes; Age in years; Height in meters; TBM =
total body mass (kg); BMI = body mass index (kg/m2); BF% = body fat percentage
(%); min = minutes; HIIE = high intensity interval exercise; HIIT = high
intensity interval training; YYIRT = Yo-Yo intermittent recovery test; CK =
creatine kinase; LDH = lactate dehydrogenase. *difference
between groups; NI = not informed
Table II
presents the biochemical variations and the results of the included studies.
The protocols were slightly different concerning the times when data were
collected in each study and the number of collections performed. Two studies
collected only pre and post-test [23,25], Aloui et
al. [25] performed pre and post measurements twice, each at a different
time of the day (morning and afternoon). Another 3 studies [21,22,24] checked
CK levels in 3 periods, Farias-Junior et al. [21] and Brandão et al. [22] in pre, 24, and 48 h, and Dorneles et al. [24] in pre, post, and 30 min. Cipryan [20] collected data in four phases: pre, post, 3,
and 24h.
All 6 studies,
each with a different exercise protocol and time of collection, evaluated CK.
Five of these studies showed a significant increase in CK [21,22,23,24,25]. Only Cipryan [20] did not observe a significant difference in
this biomarker at any time. Three studies observed a significant increase
immediately after exercise [23,24,25]. Dorneles et
al. [24] also found a significant increase 30 min after the test.
Farias-Junior et al. [21] and Brandão et al.
[22] found changes 24 and 48 hours after the work performed, with variation at
48 hours only in the 30/30 protocol.
In the studies
by Cipryan [20], Farias-Junior et al. [21],
and Brandão et al. [22], the increase in CK
peaked 24 hours after exercise, while the peak occurred in 30 minutes in Dorneles et al. [24].
As for LDH, 5 of
the 6 studies [20,22,23,24,25] found a significant increase after high-intensity
interval running, and 4 studies [20,23,24,25] observed increases only immediately
after the test. Brandão et al. [22] found a
significant increase in LDH only 24 h after exercise. Farias-Junior et al. [21]
found no significant difference in LDH at any time.
Farias-Junior et
al. [21] and Brandão et al. [22] found an
LDH peak 24 h after the protocol was performed. Dorneles
et al. [24] and Cipryan [20] observed LDH peak
immediately after exercise.
Table II - Biochemical
variations and main results

H = men; N = normal
weight; O = overweight; A = athletes; CK = creatine kinase; LDH = lactate
dehydrogenase; HIIE = high intensity interval exercise; HIIT = high intensity
interval training; MICE = moderate intensity continuous exercise; NI = not
informed. *difference between groups; #difference
between moments
Table III
presents the assessment of methodological quality, using the TESTEX tool. The
main methodological flaws observed were related to the reported randomization
criteria and the blinding of the evaluators. These items were not scored in any
of the included studies since all studies had a quasi-experimental design.
Table III - Methodological
quality of selected studies
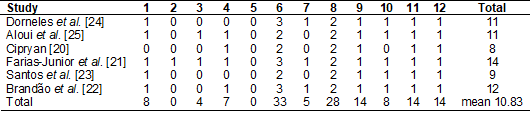
1 - Eligibility
criteria specified; 2 - Randomization specified; 3 - Allocation concealment; 4
- Groups similar at baseline; 5 - Blinding of evaluator; 6 - Withdrawals from
the study < 15%; reported adverse events; reported session attendance; 7 -
Intention-to-treat analysis; 8 - Primary and secondary between-group
statistical comparisons reported; 9- Point measures for all results; 10 -
Activity monitoring in control groups; 11 - Relative exercise intensity
remained constant; 12 - Exercise energy expenditure reported
The main sources
of bias in the present review were related to the measurement of results and
the selection of the reported result, because, according to the ACROBAT-NRSI
tool, the possibility of influencing the measurement of results due to the
non-blinding of the researchers is sufficient for the risk of bias is at least
moderate [19]. Thus, all included studies had a moderate risk of bias (Table
IV).
Table IV - Risk
of bias analysis of selected studies
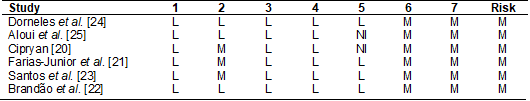
1 - Bias due to
confounding; 2 - Bias in selection of participants into the study; 3 - Bias in
measurement of interventions; 4 - Bias due to departures from intended
interventions; 5 - Bias due to missing data; 6 - Bias in measurement of
outcomes; 7 - Bias in selection of the reported result. L = low; M = moderate;
NI = not informed
Discussion
The present
systematic review described the changes in the tissue injury biomarkers (CK and
LDH) after high-intensity interval running. The heterogeneity of the methods
and the characteristics of the samples of the included studies indicate that
the results found must be analyzed with caution. It was observed that, despite
the different protocols used, four of the six studies found a significant
increase in CK and LDH concentrations simultaneously [22,23,24,25]. However, the
extent of these changes has not always occurred to the same magnitude.
The CK results
were consistent in terms of the behavior observed immediately after exercise,
since five of the six studies showed an increase in CK levels [21,22,23,24,25]. The
cause of this increase is pointed to the damage caused to the muscle fiber
structures [26], more specifically to the sarcolemma membrane [27].
Furthermore, the
changes depend on the protocol used, intensity, volume, frequency, time of
post-test collection, number, and physical conditioning of the samples [28].
Moghadam-Kia et al. [29] mention that the type and duration of exercise
are the main factors for variation in CK levels. Strenuous exercises are
responsible for the highest elevation. Gender and race also have a significant
contribution to the variation of this biomarker, with CK levels higher in men
than women and in black people when compared to white people [29,30].
The increase in
CK, observed in studies whose protocols involved running, can be explained by
the mechanism of the stretching-shortening cycle, which generates muscle
microlesion in the lower limbs during running [31]. Another explanation for the
increase in CK levels may be the characteristic of the exercises to generate
tension, which promotes muscle damage and results in the increase of this
enzyme [32]. Besides, the eccentric muscle action implies greater muscle damage
[27,30]. Such changes may take a week to return to baseline levels [33].
The CK peak
occurs 24 to 96 hours after the onset of activity [27,34,35], which was
observed in three [20,21,22] of the six included studies, although the Cipryan’s study [20] presented the interval 90% confidence,
which requires careful analysis of this result. However, as in Cerqueira et al. [36], another three studies did not
show such a pattern or did not collect at that time [23-25]. At rest, CK levels
tend to be higher in athletes when compared to healthy individuals, despite
after exercise, the increase in CK levels tends to be lower in athletes [3].
Although LDH
shows a difference in CK regarding metabolic adaptations to exercise [27], a
similar behavior was observed between the indicators of muscle damage CK and
LDH in four [22,23,24,25] of the six studies included. This finding can be explained
by the biochemical adaptation to the physical load because when CK levels
remain high, individuals also have an altered LDH [37]. As with CK, the
increase of LDH levels depends on the duration and intensity of the effort
[12]. Also, van de Vyver et al. [35] reported a strong correlation
between VO2max and the peak values of the biomarkers CK and LDH.
According to
Brancaccio et al. [34], LDH activity seems to be correlated with the
individuals’ training levels and sports performance. A short interval training
can increase the activity of glycolytic and oxidative muscle enzymes, resulting
in a slight increase in LDH. This was found in the study by Klapcińska et al.
[37], who verified that the lack of adaptation to training in untrained people
can be observed by the higher concentration of LDH after a single stimulus.
However, the levels of this biomarker showed to be higher in athletes at rest
[36,37].
Callegari et
al. [31] reported that aerobic exercise, such as running, can cause an
increase in LDH from 12 to 24 hours. Bessa et al.
[38] observed a significant increase between 3 and 6 hours after intense
exercise. As in the previous study [38], another study showed that the increase
in LDH levels, in moderate to intense physical activity, begins to be noticed
from 1 to 3 hours after the end of the exercise, with a peak of 3 to 6 hours
and returning to baseline levels in 24h [39].
Such statements
confirm the results presented by most of the studies included in the present
review and contradict Delsmann et al. [40],
who observed that the increase in LDH can occur for up to 14 days after
exercise, with the peak between the third and fourth days after stimulation.
Concomitantly, Shin et al. [41] report that CK and LDH can help as
markers for assessing the degree of muscle damage since such enzymes
demonstrate skeletal muscle deficit, muscle damage, and cell necrosis.
The present
systematic review has some limitations. The different moments of evaluation of
the biomarkers, as well as the difference in the HIIT protocols used in the
included studies, hinder a comparative analysis with greater depth. The studies
included in this systematic review were related to healthy individuals. Thus,
it is not possible to declare whether the same results would be valid for
unhealthy populations. Moreover, all studies conducted the experiment with a
small number of participants, which may have contributed to greater individual
variability. Therefore, the data evaluated needs to be observed with caution.
Conclusion
Based on the
observed evidence, the present study pointed out that the CK and LDH biomarkers
have high levels with high-intensity interval running. It was found that the
measurement of these biomarkers can be a strategic tool for assessing the
exercise load, accumulation of exercise, and intensity of physical activity,
risks, and injury degree.
More research is
needed to examine the impact of other types of exercise on inflammation. It is
important that future studies carefully evaluate the intensity associated with
the type and duration of exercise since these aspects influence inflammation
during intense exercise.
Conflict of interest
The authors declare no
conflict of interest with relevant potential.
Financing source
Supported by DECEx/Brazilian Army, EsEFEx, IPCFEx, CDE and CCFEx.
Author’s contributions
Conception and design
of the research: Sales TD, Mello DB, Romão WS, Castro
JBP, Nunes RAM, Neves EB, Vale RGS. Data collection: Sales: TD, Mello DB, Romão WS, Castro JBP, Vale RGS. Data analysis and
interpretation: Sales TD, Mello DB, Castro JBP, Nunes RAM, Neves EB, Vale RGS.
Writing of the manuscript: Sales TD, Mello DB, Romão
WS, Castro JBP, Vale RGS. Critical review of the manuscript regarding important
intellectual content: Sales TD, Mello DB, Romão WS,
Castro JBP, Nunes RAM, Neves EB, Vale RGS.
References
- Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity
interval training (HIT) and continuous endurance training for VO2max
improvements: a systematic review and meta-analysis of controlled trials. Sport
Med 2015;45(10):1469–81. doi: 10.1007/s40279-015-0365-0 [Crossref]
- Wen D, Utesch T, Wu J,
Robertson S, Liu J, Hu G, et al. Effects of different protocols of high
intensity interval training for VO2max improvements in adults: A
meta-analysis of randomised controlled trials. J Sci
Med Sport 2019;22(8):941-7. doi: 10.1016/j.jsams.2019.01.013 [Crossref]
- Cipryan L, Tschakert G, Hofmann P. Acute and post-exercise
physiological responses to high-intensity interval training in endurance and
sprint athletes. J Sport Sci Med [Internet]. 2017 [cited 2021 Aug 4];16(2):219–29.
Available from: https://pubmed.ncbi.nlm.nih.gov/28630575/
- Girard J, Feng B, Chapman C. The effects of
high-intensity interval training on athletic performance measures: a systematic
review. Phys Ther Rev 2018;23(2):151-60. doi: 10.1080/10833196.2018.1462588 [Crossref]
- Dolci F, Kilding AE, Chivers P, Piggott B,
Hart NH. High-intensity interval training shock microcycle
for enhancing sport performance: a brief review. J Strength Cond Res
2020;34(4):1188-96. doi: 10.1519/JSC.0000000000003499 [Crossref]
- Warburton DER, Bredin SSD. Health benefits of physical
activity: a systematic review of current systematic reviews. Curr Opin Cardiol 2017;32(5):541-56. doi: 10.1097/HCO.0000000000000437 [Crossref]
- Puggina
EF, Tourinho Filho H, Machado DRL, Barbanti VJ.
Efeitos do treinamento e de uma prova de triathlon em
indicadores de lesão muscular e inflamação. Rev Bras Cienc Esporte
2016;38(2):115-23. doi: 10.1016/j.rbce.2015.10.014 [Crossref]
- Brancaccio
P, Maffulli N, Limongelli
FM. Creatine kinase monitoring in sport medicine. Br Med Bull 2007;81-82: doi: 10.1093/bmb/ldm014.
- Lavender AP, Nosaka K.
Comparison between old and young men for changes in makers of muscle damage
following voluntary eccentric exercise of the elbow flexors. Appl Physiol Nutr Metab
2006;31(3):218-25. doi: 10.1139/h05-028 [Crossref]
- Khan AA, Allemailem KS, Alhumaydhi FA, Gowder SJT, Rahmani
AH. The biochemical and clinical perspectives of lactate dehydrogenase: an
enzyme of active metabolism. Endocr Metab Immune Disord Drug Targets
2020;20(6):855-68. doi: 10.2174/1871530320666191230141110 [Crossref]
- Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA,
Franck PFH, et al. IFCC primary reference procedures for the measurement of
catalytic activity concentrations of enzymes at 37 degrees C. International
Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference
procedure for the measurement of catalytic concentration of aspartate
aminotransferase. Clin Chem Lab Med 2005;40(7):725-33. doi: 10.1515/CCLM.2002.125 [Crossref]
- Brancaccio P, Lippi G, Maffulli
N. Biochemical markers of muscular damage. Clin Chem Lab Med 2010;48(6):757-67. doi: 10.1515/CCLM.2010.179 [Crossref]
- Córdova
A, Navas FJ, Lazzoli JK. Os radicais livres e o dano
muscular produzido pelo exercício: papel dos antioxidantes. Rev
Bras Med Esporte 2000 [Internet];6(5):204-8. [cited 2021 set 8]. Available from: https://www.scielo.br/j/rbme/a/6kB5p4fVyKtKMvY7JrmFHsk/?format=pdf&lang=pt
- Pedersen BK, Hoffman-Goetz L. Exercise
and the immune system: regulation, integration, and adaptation. Physiol Rev 2000;80(3):105581. doi: 10.1152/physrev.2000.80.3.1055 [Crossref]
- Page MJ, McKenzie J, Bossuyt
P, Boutron I, Hoffmann T, Mulrow CD, et al. The PRISMA
2020 statement: an updated guideline for reporting systematic reviews. BMJ
2021;372. doi: 10.1136/bmj.n71 [Crossref]
- Moola S, Munn Z, Sears K, Sfetcu
R, Currie M, Lisy K, et al. Conducting systematic
reviews of association (etiology): the Joanna Briggs Institute’s approach. Int
J Evid Based Healthc 2015;13(3):163-9. doi: 10.1097/XEB.0000000000000064 [Crossref]
- Banfi G, Colombini A, Lombardi G, Lubkowska A. Metabolic markers in sports medicine. Adv Clin Chem 2012;56:1-54. doi: 10.1016/b978-0-12-394317-0.00015-7 [Crossref]
- Smart NA, Waldron M, Ismail H, Giallauria
F, Vigorito C, Cornelissen V, et al. Validation of a
new tool for the assessment of study quality and reporting in exercise training
studies: TESTEX. Int J Evid Based Healthc
2015;13(1):9-18. doi: 10.1097/XEB.0000000000000020 [Crossref]
- Sterne JAC, Higgins JPT, Reeves BC on behalf of the
development group for ACROBAT-NRSI. A Cochrane Risk of Bias Assessment Tool:
for Non-Randomized Studies of Interventions (ACROBAT-NRSI), Version 1.0.0, 24
September 2014. [Internet] [cited 2021 Aug 4]. Available from:
http://www.bristol.ac.uk/population-health-sciences/centres/cresyda/barr/riskofbias/robins-i/acrobat-nrsi/
- Cipryan L.
IL-6, antioxidant capacity and muscle damage markers following high-intensity
interval training protocols. J
Hum Kinet 2017;56(1):139-48. doi: 10.1515/hukin-2017-0031 [Crossref]
- Farias-Junior
LF, Browne RAV, Freire YA, Oliveira-Dantas FF, Lemos TMAM, Galvão-Coelho NL, et
al. Psychological responses, muscle damage, inflammation, and delayed onset
muscle soreness to high-intensity interval and moderate-intensity continuous
exercise in overweight men. Physiol
Behav 2019;199:200-9. doi: 10.1016/j.physbeh.2018.11.028 [Crossref]
- Brandão
LHA, Chagas TPN, Vasconcelos ABS, Oliveira VC, Fortes LS, Almeida MB, et al. Physiological
and performance impacts after field supramaximal high-intensity interval
training with different work-recovery duration. Front Physiol
2020;11:1075. doi: 10.3389/fphys.2020.01075 [Crossref]
- Santos PMF, Souza LMV, Santos MB, Araújo JES, Santos
JL, Santos IB, et al. O
efeito agudo do Rast Test sobre o estresse oxidativo
e os marcadores de danos musculares em atletas jovens. J Phys
Educ 2018;29(1):e-2980. doi: 10.4025/jphyseduc.v29i1.2980 [Crossref]
- Dorneles
GP, Haddad DO, Fagundes VO, Vargas BK, Kloecker A,
Romão PRT, et al. High intensity interval exercise decreases IL-8 and
enhances the immunomodulatory cytokine interleukin-10 in lean and
overweight-obese individuals. Cytokine 2016;77:1-9. doi: 10.1016/j.cyto.2015.10.003 [Crossref]
- Aloui K, Abedelmalek S, Chtourou H, Wong
DP, Boussetta N, Souissi N.
Effects of time-of-day on oxidative stress, cardiovascular parameters,
biochemical markers, and hormonal response following level-1 Yo-Yo intermittent
recovery test. Physiol Int 2017;104(1):77-90. doi: 10.1556/2060.104.2017.1.6 [Crossref]
- Baumert P, Lake MJ, Stewart CE, Drust
B, Erskine RM. Genetic variation and exercise-induced
muscle damage: implications for athletic performance, injury and ageing. Eur J
Appl Physiol 2016;116(9):1595-625. doi: 10.1007/s00421-016-3411-1 [Crossref]
- Brancaccio P, Limongelli FM,
Maffulli N. Monitoring of serum enzymes in sport. Br J Sports Med 2006;40(2):96-7. doi: 10.1136/bjsm.2005.020719 [Crossref]
- Silva
FOC, Macedo DV. Exercício físico, processo inflamatório e adaptação: uma visão
geral. Rev Bras Cineantropom Desempenho Hum 2011;13(4):320-8. doi: 10.5007/1980-0037.2011v13n4p320 [Crossref]
- Moghadam-Kia
S, Oddis CV, Aggarwal R.
Approach to asymptomatic creatine kinase elevation. Cleve Clin J Med 2016;83(1):37-42. doi: 10.3949/ccjm.83a.14120 [Crossref]
- Koch
AJ, Pereira R, Machado M. The creatine kinase response to resistance exercise. J Musculoskelet Neuronal Interact [Internet] 2014 [cited 2021 Aug 4];14(1):68-77. Available from:
https://pubmed.ncbi.nlm.nih.gov/24583542/
- Callegari
GA, Novaes JS, Neto GR, Dias I, Garrido ND, Dani C. Creatine
kinase and lactate dehydrogenase responses after different resistance and aerobic exercise protocols. J Hum Kinet
2017;58(1):65-72. doi: 10.1515/hukin-2017-0071 [Crossref]
- Paschalis V, Koutedakis Y, Jamurtas AZ, Mougios V, Baltzopoulos V. Equal volumes of high and low intensity of
eccentric exercise in relation to muscle damage and performance. J Strength
Cond Res 2005;19(1):184-8. doi: 10.1519/R-14763.1 [Crossref]
- Kobayashi Y, Takeuchi T, Hosoi T, Yoshizaki
H, Loeppky JA. Effect of a marathon run on serum
lipoproteins, creatine kinase, and lactate dehydrogenase in recreational
runners. Res Q Exerc Sport 2005;76(4):450-5. doi: 10.1080/02701367.2005.10599318 [Crossref]
- Brancaccio
P, Maffulli N, Buonauro R, Limongelli FM. Serum enzyme monitoring in sports
medicine. Clin Sports Med 2008;27(1):1-18. doi: 10.1016/j.csm.2007.09.005 [Crossref]
- van de Vyver M, Myburgh KH.
Cytokine and satellite cell responses to muscle damage: interpretation and
possible confounding factors in human studies. J Muscle Res Cell Motil 2012;33(3-4):177-85. doi: 10.1007/s10974-012-9303-z [Crossref]
- Cerqueira É, Marinho DA, Neiva HP, Lourenço O.
Inflammatory effects of high and moderate intensity exercise – A systematic
review. Front Physiol 2020;10:1550. doi: 10.3389/fphys.2019.01550 [Crossref]
- Klapcińska B, Iskra J, Poprzecki S, Grzesiok K. The effects of sprint (300 m) running on plasma
lactate, uric acid, creatine kinase and lactate dehydrogenase in competitive
hurdlers and untrained men. J Sports Med Phys Fitness [Internet]. 2001 [cited 2021
Aug 4];41(3):306-11. Available from: https://pubmed.ncbi.nlm.nih.gov/11533559/
- Bessa
AL, Oliveira VN, Agostini GG, Oliveira RJS, Oliveira ACS, White GE, et al. Exercise
intensity and recovery: Biomarkers of injury, inflammation, and oxidative
stress. J Strength Cond Res 2016;30(2):311-9. doi: 10.1519/JSC.0b013e31828f1ee9 [Crossref]
- Lippi G, Schena F, Ceriotti F. Diagnostic biomarkers of muscle injury and
exertional rhabdomyolysis. Clin Chem Lab Med 2018;57(2):175-82. doi: 10.1515/cclm-2018-0656 [Crossref]
- Delsmann MM, Stürznickel J, Amling M, Ueblacker P, Rolvien T. Musculoskeletal laboratory diagnostics in competitive sport. Orthopade 2021. doi: 10.1007/s00132-021-04072-1 [Crossref]
- Shin K-A, Park KD, Ahn J, Park Y, Kim Y-J. Comparison of changes in biochemical markers for skeletal muscles, hepatic metabolism, and renal function after three types of long-distance running: observational study. Medicine (Baltimore) 2016;95(20):e3657. doi: 10.1097/MD.0000000000003657 [Crossref]