Rev Bras Fisiol Exerc 2021;20(6):644-54
doi: 10.33233/rbfex.v20i6.4859
STUDY PROTOCOL
High-intensity
interval training versus moderate-intensity continuous training on cardiac
autonomic control in hypertensive patients
Treinamento intervalado de alta
intensidade versus treinamento contínuo de moderada intensidade no controle
autonômico cardíaco de pacientes hipertensos
Fabrício Olinda de Souza Mesquita1, Victor
Ribeiro Neves1, Eduardo Seiji Numata Filho1, Sérgio Rodrigues Moreira2,
Aparecida Maria Catai3, Paulo Adriano Schwingel1
1Universidade de Pernambuco (UPE),
Petrolina PE, Brazil
2Universidade Federal do Vale do São
Francisco (UNIVASF), Petrolina, PE, Brazil
3Universidade Federal de São Carlos
(UFSCAR), São Carlos, SP, Brazil
Received: July 23, 2021; Accepted:
November 22, 2021.
Correspondence: Fabrício Olinda de Souza Mesquita, Av.
José de Sá Maniçoba, s/n - Centro, 56304-205 Petrolina PE
Fabrício Olinda de Souza
Mesquita: fabricioolinda@hotmail.com
Victor Ribeiro Neves:
victor.neves@upe.br
Eduardo Seiji Numata Filho:
dunumata07@gmail.com
Sérgio Rodrigues
Moreira: serginhocapo@gmail.com
Aparecida Maria Catai:
mcatai@ufscar.br
Paulo Adriano Schwingel: paulo.schwingel@upe.br
Abstract
Aim:
This study aims to verify and compare the acute and chronic effects of
high-intensity interval training and moderate-intensity continuous training
sessions on the linear and nonlinear heart rate variability indexes, responses
of blood pressure, aerobic power, aerobic capacity, and quality of life from patients
with systemic arterial hypertension. Methods: Controlled, randomized
clinical trial with intention-to-treat analysis. Non-alcoholic, non-diabetic
patients between 18 and 60 years of age, with a diagnosis of prehypertension or
stage I hypertension for at least 12 months and with controlled blood pressure
levels. Conclusion: This protocol study intends to show that
high-intensity interval exercise in controlled hypertensive patients with low
cardiovascular risk has a greater hypotensive effect, as well as an increase in
vagal modulation on the heart.
Keywords:
hypertension; high-intensity interval training; blood pressure monitoring,
ambulatory; cardiorespiratory fitness.
Resumo
Objetivo: O presente estudo tem como objetivo
verificar e comparar os efeitos agudos e crônicos do treinamento intervalado de
alta intensidade e do treinamento contínuo de moderada intensidade sobre os
índices lineares e não lineares de variabilidade da frequência cardíaca,
respostas da pressão arterial, potência aeróbia, capacidade aeróbia e qualidade
de vida de pacientes com hipertensão arterial sistêmica. Métodos: Ensaio
clínico randomizado e controlado com análise por intenção de tratar. Pacientes
entre 18 e 60 anos, não etilistas e não diabéticos, com diagnóstico de pré-hipertensão ou hipertensão estágio I há pelo menos 12
meses, com níveis pressóricos controlados. Conclusão: Este protocolo de
estudo pretende mostrar que o exercício intervalado de alta intensidade em
hipertensos controlados, com baixo risco cardiovascular, tem um maior efeito
hipotensor, bem como aumenta a modulação vagal no coração.
Palavras-chave: hipertensão; treinamento intervalado
de alta intensidade; monitorização ambulatorial da pressão arterial; aptidão
cardiorrespiratória.
Introduction
Several
studies have focused on comparing the effects of high-intensity interval
training (HIIT) versus moderate-intensity continuous training (MICT) in
hypertensive populations. Two elegant systematic reviews with meta-analyses
[1,2] have not observed a superior effect of HIIT to MICT in systolic and
diastolic blood pressures reduction. On the other hand, HIIT favored an
improvement in aerobic fitness with the greatest increase in VO2max
[1,2].
It is
well established in the literature that just one aerobic exercise session can
reduce blood pressure recovery when compared to pre-intervention moments. In
addition, aerobic exercise promotes beneficial adaptations in other health
variables, such as aerobic fitness [3,4]. This acute response is positively related
to the chronic effect of aerobic training in blood pressure [2,5]. However,
this association has only been clinically observed, which emphasizes the need
for investigating outpatient blood pressure levels [6], which may have
minimized the effects of the observer (white coat syndrome) and better reflect
the actual blood pressure values in daily activities [7].
If,
on the one hand, the risk for fatal and non-fatal cardiovascular events may
increase due to the increase in exercise intensity [8,9], on the other hand,
the safety and risk-benefit of prescribing HIIT in individuals with impairment
chronic cardiac failure have already been demonstrated by the decrease in
premature ventricular contractions and increase in vagal tone after a session
of high-intensity interval exercise [10]. Previous studies on the effects of
HIIT on the autonomic nervous system in hypertensive patients remain scarce in
the literature. Since autonomic dysfunction has been demonstrated in this
population, it is speculated that both HIIT and MICT may improve cardiac
autonomic control and as such treat systemic arterial hypertension (SAH) and
its progression [8].
Besides,
HIIT promoted an increase in vagal tone after a session of high-intensity
interval exercise [10] and decrease arrhythmic events in a 24-h post-training
period in heart failure patients. In addition, studies involving hypertensive
patients have not reported adverse events during HIIT programs [2]. However,
further investigations are still needed to assess the autonomic control and
potential cardiac arrhythmias after a session of intense aerobic exercise in
hypertensives.
Therefore,
the main purpose of this study is to assess and compare the effects of HIIT and
MICT on the cardiac autonomic nervous system in patients with hypertension.
Additionally, the secondary aims are 1) to assess HIIT effects on blood
pressure, aerobic capacity, aerobic power, and quality of life; 2) to assess
acute responses of HIIT on heart rate variability after one exercise session;
3) to identify potential adverse cardiovascular effects of HIIT in patients
with hypertension.
Methods
Study design
This
is a controlled, randomized clinical trial with intention-to-treat analysis
[11] that will be carried out at the Exercise Physiology Laboratory, located in
the Department of Physical Education (CEFIS) of the Universidade
Federal do Vale do São Francisco (UNIVASF) and in the Human Performance
Research Laboratory at Petrolina Campus of the Universidade de Pernambuco (UPE).
This
study was approved by the Brazilian Clinical Trials Records (REBEC)
[Clinicaltrials.gov, REBEC registration: RBR-2fdkw3) and the Research Ethics
Committee (CEP) from UPE (CAAE registration: 69902817.5.0000.5207) and
conducted according to the principles outlined in the Declaration of Helsinki.
Only participants who provide written informed consent will be enrolled in the
study.
Sample selection
The
volunteers will be referred to a cardiological evaluation, which includes a
cardiac stress test on a treadmill and lipid blood test panel plus fasting
glucose. In sequence, an anamnesis will be performed by a researcher to obtain
personal information, alcohol intake behavior, drug use, tobacco use behavior,
and physical activity. Height, body weight, frequency of daily administration
of blood pressure-lowering drugs, and other medicines will be recorded. Current
alcohol use will be assessed as the frequency of alcohol consumption and the
amount of alcohol consumed per drinking day. The frequency (in days) and
duration (in minutes) of physical activities will be self-reported.
It
will not be included patients with secondary hypertension, ischemic heart
disease, heart failure, complex arrhythmias, tachyarrhythmia, and/or atrial
fibrillation diagnosed by a cardiologist during the exercise stress test. Will
also not be included volunteers with body mass index (BMI) ≥ 35 kg.m-2,
who regularly practiced physical activity at least one day per week during the
previous three months (sedentary lifestyle), frequent drinkers (a history of
ethanol intake > 20 g/day or > 140 g/week), pregnant women, and/or
diabetics (fasting blood sugar level of 126 mg/dL or higher).
The
inclusion criteria adopted will be adults from 18 to 60 years old with a
diagnosis of prehypertension or stage 1 hypertension for at least 12 months
with blood pressure levels controlled by antihypertensive drugs with no changes
in the medication dosage in the previous three months.
It
will be excluded those who present any osteoarticular, cardiovascular, and/or
metabolic changes that prevented them from continuing in the study or the
patients who change the medication.
Hereafter,
volunteers will proceed to the pre-intervention evaluations: 1) HRV in the
supine and orthostatic positions (20 minutes), and the 24-hour using a Holter
electrocardiogram; 2) ambulatory blood pressure monitoring for 24 hours; 3)
aerobic capacity and aerobic power through the incremental test (TI); 4)
quality of life by the SF-36 questionnaire. It should
be noted that all assessments will be standardized to take place in the
morning. Likewise, the evaluation schedule for everyone will be maintained throughout
the study (Figure 1).
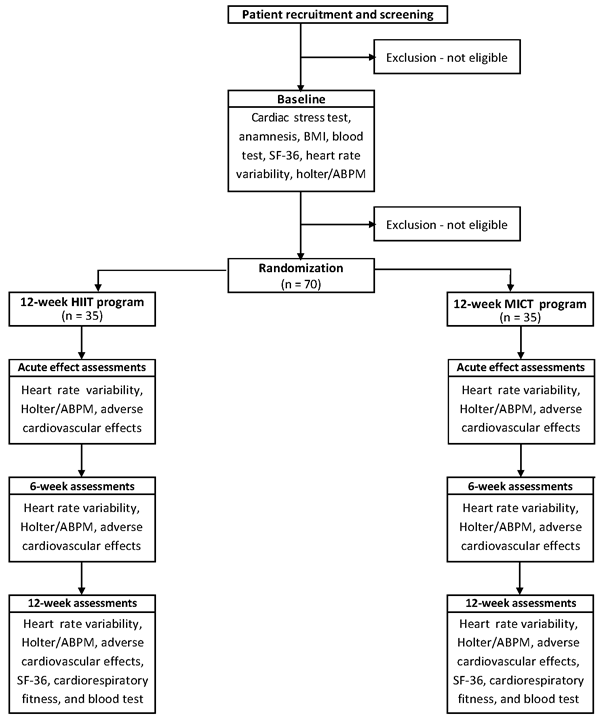
HIIT
= high-intensity interval training; MICT = moderate-intensity continuous
training; BMI = body mass index; ABPM = ambulatory blood pressure monitoring;
SF-36 = Medical Outcomes Study 36 – Item Short-Form Health Survey
Figure 1 - Schedule
of enrolment, interventions, and assessments
Sample
randomization will be undertaken at the end of pre-intervention evaluations
through a hidden sequence of blocks randomly exchanged and stratified by sex,
generated by an independent researcher using the random numbers from Excel®
(Microsoft Corporation, Redmond, WA, United States of America [USA]). The study
will be conducted by researchers who will be blinded to: 1) conducting
evaluations of outcome measures, and 2) carrying out statistical procedures. In
addition, all participants will be instructed not to disclose which of the
groups they have been allocated.
Outcome measures
The
primary outcome of the trial is to assess and compare the effects of 12-week
HIIT intervention on the cardiac autonomic control by heart rate variability
indexes in individuals with prehypertension or with stage 1 hypertension.
Secondary outcomes are changes in cardiorespiratory fitness, blood pressure
levels, maximal aerobic power, and adverse cardiovascular effects.
Procedures
Heart rate
variability indexes
Before
this assessment, patients will be instructed not to consume alcoholic and/or
caffeinated beverages 24 hours before assessments. In addition, all volunteers
will have to present respiratory rates within the range of the high frequency
band (from 0.15 to 0.40 Hz), that is, greater than 9 breaths per minute.
The
patients will remain at rest for 10 minutes. After that, R–R intervals (RRi) will be recorded for 10 minutes in the supine position
and 10 minutes in the orthostatic position. At this time, RRi
are recording by Wincardio electrocardiogram recorder
(MICROMED Biotecnologia Ltda., Brasília, DF, Brazil)
with 12 simultaneous leads placed on the patient's chest at specific points to
a receiver and then to the computer for further analysis.
HRV
analysis in the frequency domain (spectral analysis) will be performed using an
autoregressive model [12], and the low-frequency (LF: from 0.04 to 0.15 Hz) and
HF (from 0.15 to 0.40 Hz) bands will be obtained. The sequence of RRi with 256 beats and with the greatest stability will be
selected for each subject. This sequence will be used for both linear and
nonlinear analysis. In addition, the mean and variance of the RRi will also be calculated according to the Catai checklist [13].
The
normalization of the indices will consist of dividing a given power of each
spectral component (LFabs or HFabs)
by the total power minus the very low frequency (VLF: < 0.04 Hz), and then
multiplying the ratio by 100 [14,15]. These spectral components will be
expressed in absolute units (LFabs and HFabs) and normalized units (LFun
and HFun) [16].
For nonlinear analysis, the RRi will be transformed into a sequence of symbols
(numbers) ranging from 0 to 5. Then, the patterns of a sequence of 3 beats will
be determined. The pattern distribution will be calculated using Shannon's
entropy (SE). This index describes the distribution of patterns. SE is high if
the distribution is flat (all patterns are identically distributed, and the
series carries as much information as possible). On the other hand, SE is low
if a subset of patterns is more common, while other patterns are absent or
infrequent [15,16].
To
perform symbolic analysis (SA), all patterns will be grouped into four families
as follows: (a) patterns without variation (0V: all symbols are equal, i.e.
2,2,2 or 4,4,4) ; (b) patterns with one variation (1V: 2 consecutive symbols
are the same and one symbol is different, i.e. 4.2.2 or 4.4.3); (c) patterns
with two similar variations (2LV: 3 symbols that form an ascending or
descending ramp, i.e. 5.4.2 or 1.3.4); (d) two different variations (2ULV: 3
symbols that form a peak or a tail, i.e. 4,1,2 or 3,5,3). The rate of
occurrence of each pattern is defined as 0V%, 1V%, 2LV%, and 2ULV%, and 0V% and
2ULV% may be considered markers of sympathetic and vagal modulation,
respectively.
Furthermore,
the conditional entropy (CE) will also be assessed through the complexity index
(CI). This index will be normalized by the SE of the RRi
series to obtain a normalized CI (NCI), thus expressing the complexity in terms
of dimensional units, ranging from 0 (null information) to 1 (maximal
information). The higher the CI and the NCI, the greater the complexity and the
less regular the series [16].
24-hour ambulatory
blood pressure monitoring and Holter monitoring
The
simultaneously 24-hour ambulatory blood pressure monitoring (ABPM) and 24-hour
Holter monitoring will be performed using the oscillometric
device CardioMapa (CARDIOS, São Paulo, SP, Brazil).
Measurements will be taken every 20 minutes during the waking period (6 am to
11 pm), and every 30 minutes during the sleep period (11 pm to 6 am). The time
between bedtime and getting up will be considered as a sleep period, which will
be noted down in the diary of each volunteer.
After
automatic scanning, an export analyst carefully will edit all the recordings.
Annotated RRi time series will be finally transferred
to a personal computer and will process according to previously described
criteria [14] to identify ectopic beats, arrhythmic events, and artifacts
[17,18].
Cardiopulmonary
exercise testing
An
incremental protocol [19] on a mechanically braked cycle ergometer Biotec 2100 (CEFISE®, São Paulo, SP, Brazil) will be used
for the cardiopulmonary exercise testing (CPET). All individuals will be
instructed not to consume stimulating and/or alcoholic beverages on the day
before the test. CPET will start with a load of 30 Watts and the cadence was
maintained at 60 ± 5 revolutions per minute (rpm). There will be increments of
15 Watts of load at each stage, lasting 2 minutes, until the maximum voluntary
exhaustion of the subject [19]. The final stage will be defined by the
completion of at least 51% of the total time.
Pulmonary
ventilation, oxygen consumption, and heart rate will be monitored and recorded
continuously using an ergospirometer Fitmate Pro (COSMED Srl., Rome,
Italy). Peak oxygen consumption will be defined as the highest value reached in
the final 20 seconds of the test.
Quality of life
assessment
The
Brazilian version of the validated SF-36 quality of life questionnaire [20]
will be administered. The questionnaire is a generic multidimensional 36-item
instrument for assessing the quality of life, encompassing eight dimensions or
components: functional capacity, physical aspects, pain, general health status,
vitality, social aspects, emotional aspects, and mental health. The
questionnaire score ranges from 0 to 100, in which 0 corresponds to the worst
general health status and 100 to the best health status [20].
Sample size
Given
the pragmatic nature of the trial, a reduction of 3.2 mmHg in systolic blood
pressure in the HIIT group compared to the MICT group is considered a
clinically relevant difference according to a systematic review with
meta-analysis of studies on the effects of endurance training, resistance
training, and combined training in the ambulatory blood pressure [21]. In the
present study, a sample size of 35 patients in each group will be sufficient to
detect this difference according to the G*Power software
(Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) assuming a
standard deviation of 3.8 mmHg, a power of 80%, and a significance level of 5%.
The assumed standard deviation is based on observations from Cornelissen and
Smart [21].
Experimental
intervention – training protocol
Training
with their respective exercise sessions will be carried out in a controlled
temperature environment (22 ± 1°C), with a relative humidity of 55 ± 5%. In
both training groups, the exercise sessions will be performed on the same
mechanically braked cycle ergometer Biotec 2100
(CEFISE®) twice a week, for 12 weeks. Additionally, a cadence of 60 ± 5 rpm
will be maintained for both training protocols.
HIIT
sessions will start with a five-minute warm-up with no load. During the entire
study period, 10 one-minute series will be performed at 80 to 90% of PMAX, with
a two-minute interval between the series at 40% of PMAX. All sessions in the
different phases of the training will last 30 minutes.
Likewise,
MICT sessions will start with a five-minute warm-up without load. The load used
will be adjusted to 50% of the PMAX during the entire training period. Exercise
volumes of HIIT and MICT will be equalized according to Buchheit
and Laursen [22]. Table I shows the training
periodization.
Table I - Periodization
of high-intensity interval training (HIIT) and moderate-intensity continuous
training (MICT) individuals with essential arterial hypertension classified as
borderline or stage I
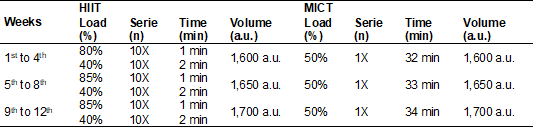
The
training volume was calculated using the equation: volume = loads X series X
times [33]; a.u.: arbitrary unit
Statistical
analysis
The
data will be double entered into the SPSS software (SPSS Inc., Chicago, IL,
USA) with an automatic amplitude and consistency checking. All statistical
analysis will be carried out in the SPSS by a blinded statistician. The
statistical analysis will be descriptive with the Shapiro–Wilk assessing the
normality of the data. Categorical variables will be described in absolute and
relative frequencies (number and percentages) and the continuous variables as
mean ± standard deviation in a parametric distribution or as median (first
quartile – third quartile) in case of non-Gaussian distribution. The
homogeneity of the data will be verified by Levene’s
test.
The
primary endpoint for the statistical analysis is the mean change in heart rate
variability indexes from baseline to 12 weeks of follow-up. The primary
endpoint will be compared between intervention arms using a general linear
model with the treatment group, baseline HRV, and 6-week HRV fitted as
covariates, and 12-week HRV as the dependent variable. The linear model will be
adjusted for the continuous covariate age and the categorical covariate sex.
Further adjustment variables may be investigated as part of the exploratory
analysis.
The
secondary endpoints will be analyzed using the same covariates as the primary
endpoint analysis. Likewise, the differences between groups in terms of
continuous secondary outcome measures will be assessed with the same
statistical model as the primary outcome analysis. Differences between
treatment arms for binary, unordered categorical and ordinal secondary outcome
variables will be analyzed using logistic regression, multinomial logistic
regression, and proportional odds models, respectively.
The
primary and secondary endpoints analyses will be two-tailed with the alpha
level set at 5%. Effect sizes, 95% confidence intervals, and statistically and
clinically significant differences will be calculated. All secondary analyses
will be exploratory if a non-significant result is obtained from the primary
analysis and, whenever reported, the failure to achieve a significant result in
the primary analysis will be declared. All analyses will be performed on an
intention-to-treat basis.
All
data will be summarized and reported following the Consolidated Standards of
Reporting Trials (CONSORT) guideline. No formal interim analyses are
anticipated.
Discussion and
expected results
This
protocol study intends to show that high-intensity interval exercise in
controlled hypertensive patients with low cardiovascular risk, has a greater
hypotensive effect, as well as an increase in vagal modulation on the heart. In
addition, it is expected that the degree of blood pressure reduction, right
after an acute exercise session, is related to the magnitude of the blood
pressure change, especially after the application of high-intensity interval
training.
In addition,
the basis of these results, other studies may be carried out in different
clinical conditions and training modalities, to provide health professionals
with information that identifies the degree of safety in performing aerobic
exercises with a higher degree of intensity and in populations with clinical or
pathological limitations, such as arterial hypertension.
Besides
that, Pescatello et al. [23] recently
published a systematic review involving adults with and without hypertension.
They included 17 meta-analyses and one systematic review. Of these, six were on
the effect of aerobic exercise on blood pressure and reported a significant
reduction in systolic and diastolic blood pressure. However, none of the
systematic reviews included assessed the effects of HIIT. Moreover, there
appears to be no published meta-analysis on the effects of HIIT in hypertensive
patients. Furthermore, the meta-analysis technique minimizes subjectivity by
standardizing the treatment effects of relevant studies according to effect
size, by pooling results, and by analyzing the resulting data [23]. Then more
studies are needed on the efficacy and safe of alternative exercise protocols
on health in varied clinical situations.
Dissemination
and impact
Throughout
the clinical trial, media outlets (including social media) will be informed of
progress, and the experiences gained will be presented at national conferences.
On completion, the study results will be published in peer-reviewed journals
and presented at scientific meetings.
Conflicts
of interest
No
potential conflicts of interest relevant to this article have been reported.
Funding
This study
was financed in part by the
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001; the Fundação de Amparo à Ciência e Tecnologia do Estado de
Pernambuco (FACEPE) [grant numbers
APQ-0246-4.06/14, APQ-0789-4.08/17]; and the Conselho Nacional de Desenvolvimento Científico e
Tecnológico (CNPq) [grant number
426706/2016-1].
Author´s
contributions
Study
conception and design: Mesquita FOS, Neves
VR, Catai AM, Schwingel PA;
Draft manuscript preparation: Mesquita FOS, Neves VR, Numata Filho ES, Catai AM; Review
and editing the manuscript: Neves VR, Numata
Filho ES, Schwingel PA; Analysis and
interpretation of results: Moreira SR, Schwingel
PA; Critical review and manuscript revision: Moreira SR, Catai AM; Project administration: Neves VR; Project
supervision: Neves VR, Schwingel PA; Funding
acquisition: Neves VR, Schwingel PA; All authors
reviewed and approved the final version of the manuscript.
References
- Leal JM, Galliano LM, Del Vecchio FB. Effectiveness
of high-intensity interval training versus moderate-intensity continuous
training in hypertensive patients: a systematic review and meta-analysis. Curr Hypertens Rep 2020;22(3):26. doi: 10.1007/s11906-020-1030-z [Crossref]
- Costa EC, Hay JL, Kehler DS, Boreskie KF, Arora RC, Umpierre D, et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: a systematic review and meta-analysis of randomized trials. Sport Med 2018;48(9):2127-42. doi: 10.1007/s40279-018-0944-y [Crossref]
- Warburton DER, Bredin SSD. Health benefits of physical activity. Curr Opin Cardiol 2017;32(5):541-556. doi: 10.1097/HCO.0000000000000437 [Crossref]
- Cardoso Junior CG, Gomides RS, Queiroz ACC, Pinto LG, Lobo FS, et al. Acute and chronic effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics 2010;65(3):317-25. doi: 10.1590/S1807-59322010000300013 [Crossref]
- Hecksteden A, Grütters T, Meyer T. Association between postexercise hypotension and long-term training-induced blood pressure reduction. Clin J Sport Med 2013;23(1):58-63. doi: 10.1097/JSM.0b013e31825b6974 [Crossref]
- Andrade DM, Amaral JF, Trevizan PF, Toshi-Dias E, Silva LP, Laterza MC, et al. Anxiety increases the blood pressure response during exercise. Mot Rev Educ Física 2019;25(3). doi: 10.1590/s1980-6574201900030016 [Crossref]
- Segre CA, Ueno RK, Warde KRJ, et al. White-coat hypertension and normotension in the League of Hypertension of the Hospital das Clínicas, FMUSP: prevalence, clinical and demographic characteristics. Arq Bras Cardiol 2003;80(2). doi: 10.1590/S0066-782X2003000200001 [Crossref]
- Lima AHRA, Forjaz CLM, Silva GQM, Lima APA, Lins Filho OL, Cardoso Júnior CG, et al. Effect of rest interval on cardiovascular responses after resistance exercise. Mot Rev Educ Física 2013;19(2):252-260. doi: 10.1590/S1980-65742013000200002 [Crossref]
- Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events. Circulation 2007;115(17):2358-68. doi: 10.1161/CIRCULATIONAHA.107.181485 [Crossref]
- Guiraud T, Labrunee M, Gaucher-Cazalis K, Despas F, Meyer P, Bosquet L, et al. High-Intensity interval exercise improves vagal tone and decreases arrhythmias in chronic heart failure. Med Sci Sport Exerc 2013;45(10):1861-7. doi: 10.1249/MSS.0b013e3182967559 [Crossref]
- Soares I, Carneiro AV. Intention-to-treat
analysis in clinical trials: principles and practical importance. Rev Port Cardiol [Internet]. 2002 [cited 2021 Nov 22];21(10):1191-8.
Available from: https://pubmed.ncbi.nlm.nih.gov/12522981/
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986;59(2):178-193. doi: 10.1161/01.RES.59.2.178 [Crossref]
- Catai AM, Pastre CM, Godoy MF, Silva E, Takahashi ACM, Vanderlei LCM. Heart rate variability: are you using it properly? Standardisation checklist of procedures. Brazilian J Phys Ther 2020;24(2):91-102. doi: 10.1016/j.bjpt.2019.02.006 [Crossref]
- Heart rate
variability: standards of measurement, physiological interpretation and
clinical use. Task Force of the European Society of Cardiology and the North
American Society of Pacing and Electrophysiology. Circulation [Internet] 1996
[cited 2021 Nov 22];93(5):1043-65. Available from:
https://pubmed.ncbi.nlm.nih.gov/8598068/
- Guzzetti S, Marchi A, Bassani T, Citerio G, Porta A. Univariate and bivariate symbolic analyses of cardiovascular variability differentiate general anesthesia procedures. Physiol Meas 2015;36(4):715-726. doi: 10.1088/0967-3334/36/4/715 [Crossref]
- Porta A, Baselli G, Liberati D, Montano N, Cogliati C, Gnecchi-Ruscone, et al. Measuring regularity by means of a corrected conditional entropy in sympathetic outflow. Biol Cybern 1998;78(1):71-8. doi: 10.1007/s004220050414 [Crossref]
- Maestri R, Pinna GD, Accardo A, Allegrini P, Balocchi R, D'Addio G, et al. Nonlinear indices of heart rate variability in chronic heart failure patients: redundancy and comparative clinical value. J Cardiovasc Electrophysiol 2007;18:425-33. doi: 10.1111/j.1540-8167.2007.00728 [Crossref]
- Maestri R, La Rovere MT, Porta A, Pinna GD. Sympathetic neurohormonal correlates of linear and symbolic dynamics heart rate variability indexes in chronic heart failure. Computers in Cardiology 2008;49-52. doi: 10.1109/CIC.2008.4748974 [Crossref]
- Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J 2007;83(985):675-682. doi: 10.1136/hrt.2007.121558 [Crossref]
- Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Tradução para a língua portuguesa e
validação do questionário genérico de avaliação de qualidade de vida SF-36
(Brasil SF-36). Rev Bras Reumatol [Internet]
1999 [cited 2021 Nov 22];39:143-50. Available from:
http://www.scielo.br/pdf/csc/v16n7/10.pdf
- Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013;2(1). doi: 10.1161/JAHA.112.004473 [Crossref]
- Buchheit M, Laursen PB. High-Intensity Interval Training, solutions to the programming puzzle. Sport Med 2013;43(10):927-54. doi: 10.1007/s40279-013-0066-5 [Crossref]
- Pescatello LS, Buchner DM, Jakicic JM, Powel KE, Kraus WE, Bloodgood B, Campbell WW, et al. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc 2019;51(6):1314-23. doi: 10.1249/MSS.0000000000001943 [Crossref]
