Rev Bras Fisiol Exerc 2021;6:604-17
doi: 10.33233/rbfex.v20i6.4878
ORIGINAL ARTICLE
Effects of
different blood flow restriction pressure levels on muscular hemodynamics
Efeito de diferentes níveis de restrição
de fluxo sanguíneo sobre a hemodinâmica muscular
Ramon Franco Carvalho1, Paulo Sergio Chagas Gomes1, Márcio Lopes Fernandes
Júnior2, Claudia de Mello
Meirelles3
1Universidade do Estado do Rio de Janeiro
Rio de Janeiro, Brazil
2Universidade Estácio Sá, Campus Duque de
Caxias 2, Duque de Caxias, Rio de Janeiro, Brazil
3Escola de Educação Física do Exército,
Rio de Janeiro, RJ, Brazil
Received: August 11, 2021; Accepted:
December 2, 2021.
Correspondence: Paulo Sergio Chagas Gomes,
Universidade do Estado do Rio de Janeiro, Instituto de Educação Física e
Desportos, Rua São Francisco Xavier, 524, 8o Andar, Bloco F, Sala 8104,
Maracanã, 20550-900 Rio de Janeiro RJ
Ramon
Franco Carvalho:prof.ramonfranco@gmail.com
Paulo Sergio Chagas Gomes: paulo.gomes@uerj.br
Márcio Lopes Fernandes
Júnior: marcio.eefd.gmail.com
Abstract
Introduction:
Resistance exercise with blood flow restriction (BFR) is an effective
method to promote muscle strength gains and hypertrophy. However,
little is known about the
effects of different BFR levels on hemodynamic responses. Objective: To
verify whether the different blood flow restriction pressures applied to the
upper limb cause acute changes in vascular microcirculation in young, healthy
male adults. Methods: Ten young male visited the laboratory on four
occasions. In the first visit, after 10-min rest in supine position, the
brachial artery occlusion pressure (AOP) was identified with a Doppler
ultrasound. Thereafter, the participants were submitted to a protocol
consisting of 1 min for baseline measurements, 2 min of BFR, and 2 min after
cuff deflation. It was used a cuff placed on the proximal portion of the
forearm and inflated with pressures equivalents to 30% (30BFR), 50% (50BFR) 80%
(80BFR), or 100% (100BFR) of the AOP in a random order in separate days.
Measurements of tissue saturation index (TSI), oxyhemoglobin, deoxyhemoglobin,
and total hemoglobin were collected continuously using near-infrared
spectrometry. Results: A two-way ANOVA with repeated measures
demonstrated: 1) a significant decrease in TSI in all conditions, with higher
decay in 100BFR; 2) a significant increase in oxyhemoglobin in all conditions,
but 100BFR; 3) a similar increase in deoxyhemoglobin in all conditions; 4) a
significant increase in total hemoglobin in all conditions, mainly in both
30BFR and 50BFR. Conclusion: The relative pressures adopted demonstrated
that the hemodynamic changes do not occur linearly with the pressure level
imposed by the inflated cuff.
Keywords:
spectroscopy, near-infrared; vascular closure devices; resistance training.
Resumo
Introdução: O exercício contrarresistência
com restrição do fluxo sanguíneo (RFS) é um método eficaz para ganho de força e
hipertrofia muscular. No entanto, pouco se sabe sobre os efeitos dos diferentes
níveis de RFS nas respostas hemodinâmicas. Objetivo: Verificar se as
diferentes pressões de restrição ao fluxo sanguíneo aplicadas no membro
superior causam alterações na microcirculação vascular em adultos jovens
saudáveis do sexo masculino. Métodos:
Dez jovens do sexo masculino
visitaram o laboratório em quatro ocasiões. Na primeira
visita, após 10 min de
repouso em decúbito dorsal, a pressão de oclusão
da artéria braquial (POA) foi
identificada através de ultrassom com Doppler. Posteriormente,
os participantes
foram submetidos a um protocolo que consistia de 1 min para as medidas
basais,
2 min de RFS e 2 min após a liberação da
restrição sanguínea. Foi utilizado um
manguito colocado na porção proximal do antebraço
e inflado com pressões
equivalentes a 30% (30RFS), 50% (50RFS) 80% (80RFS) ou 100% (100RFS) do
POA em dias separados. As medições do índice
de saturação do tecido
(IST), oxihemoglobina, desoxihemoglobina
e hemoglobina total foram coletadas continuamente usando espectrometria de
infravermelho próximo. Resultados: Uma ANOVA de duas vias com medidas
repetidas demonstrou 1) uma diminuição significativa no IST em todas as
condições, com maior queda em 100RFS; 2) um aumento significativo na oxihemoglobina em todas as condições, exceto 100RFS; 3) um
aumento semelhante na desoxihemoglobina em todas as
condições; 4) um aumento significativo na hemoglobina total em todas as
condições, principalmente em 30RFS e 50RFS. Conclusão: As pressões
relativas adotadas demonstraram que as alterações hemodinâmicas não ocorrem
linearmente com o nível de pressão imposto pelo manguito insuflado.
Palavras-chave: espectroscopia de luz próxima ao
infravermelho; dispositivos de oclusão vascular; treinamento de força.
Introduction
Resistance
exercise (RE) with restricted blood flow (BFR) is an effective method to
promote strength gains [1,2,3] as well as muscle hypertrophy [2,4,5]. This method
consists of using an inflated cuff at the proximal extremity of the limbs
during the performance of an activity with relatively low resistance overload,
ranging from 10 to 50% of 1RM [6,7,8].
The
purpose of restricting the influx of arterial blood to the limb is to cause a
more significant metabolic stress and stimulate the mechanisms of muscle
hypertrophy, such as additional recruitment of motor units, cell swelling, the
release of anabolic hormones, altered production of myokines, and reactive
oxygen species [9,10,11]. Although the magnitude of the responses to strength
gains are lower than the ones obtained with traditional high resistive loads
strength training routines, resistance training with blood flow restriction
(BFR) may be a more appropriate strategy in populations that are unable to
mobilize high overload, such as the elderly [12], and people recovering from
musculoskeletal injury or surgery [13].
When
inflating a cuff in the proximal region of the upper or lower limb, venous
blood is easily occluded, generating blood storage in the venules and thus
preventing the removal of metabolites from muscle contraction. This procedure
can prevent venous blood return to the limb but still allow the entry of
arterial blood, even if in a limited way [14].
Understanding
the impact of different percentages of blood flow restriction on muscle
hemodynamics can help clarify the best relationship between metabolic stress
and the lowest health risk associated with blood flow restriction [14]. Also,
the scientific literature indicates that high-pressure loads promote a higher
level of discomfort [15].
Previous
studies [16,17] carried out in healthy young subjects at rest observed that the
reduction in blood flow occurs in a staggered and non-linear manner due to
increased pressure load. Using ultrasound in the Doppler mode, Mouser et al.
[17] observed that 10% of the pressure at the artery occlusion pressure (AOP)
applied by a cuff is enough to significantly reduce absolute and relative blood
flow speed in the brachial artery when compared to resting condition. This flow
reduction remained similar until 40% of the AOP when a further significant drop
in blood flow was observed and remained up to 80% (absolute blood flow) or 90%
(relative blood flow) when the last phase of fall occurred. Despite the
importance of this finding in blood flow, the study, as mentioned earlier, did
not observe the impact on hypoxia. The literature has shown that the
intracellular deviation of blood plasma and cellular hypoxia generated by flow
restriction significantly influences the mechanisms associated with increased
muscle strength and hypertrophy [18].
Muscle
hemodynamics measurements can also be performed by near-infrared spectroscopy
(NIRS), which is widely used in research to monitor acute and chronic muscle
perfusion changes under different settings [19].
Kilgas et al. [20] showed that 30 seconds under BFR
did not change muscle hemodynamics at pressures lower than 60% AOP, assessed by
a NIRS probe placed on the forearm of ten healthy men. Less is known about
higher periods of BFR, as employed in typical resistance exercise protocols.
Blood
flow reduction seems to occur staggered and not linear or parallel by increased
pressure levels exerted externally by a cuff. With this shortcoming in mind, it
is necessary to identify the impact of different pressure level ranges on local
hemodynamic responses, especially in cell hypoxia. This knowledge may
contribute to a better understanding of the physiological responses, allowing a
safer and more efficient prescription method.
Thus,
the present study aimed to verify whether the different blood flow restriction
pressures applied to the upper limb cause acute changes in vascular
microcirculation in young, healthy male adults.
Methods
Study sample
Ten
young college male students volunteered for the present study (age: 26 ± 5
years, biceps skinfold: 3.4 ± 1.1 mm, systolic blood pressure: 122.9 ± 7.1
mmHg, diastolic blood pressure: 81, 4 ± 7.5 mmHg, resting heart rate: 69.3 ±
5.7 bpm; body mass index: 24.7 ± 1.1 kg/m²). All participants were normotensive
and healthy based on the Physical Activity Readiness Questionnaire (Par-Q)
evaluation, and nobody was involved in any systematic physical training practice
in the last six months. All of them signed the informed consent form before
starting the tests. The Research Ethics Committee of the President Antônio
Carlos University approved this study (CAAE: 83463517.7.0000.5156), based on
the principles of the Declaration of Helsinki.
Study design
The
study was characterized by a randomized controlled trial model, and
participants attended the research laboratory on four separate occasions with
two to seven-day between trial intervals. All participants were instructed not
to consume any drink or food, like caffeine and alcohol, that would affect
hemodynamic responses and not to practice any physical activity 24 hours before
the test. Also, all visits took place within the same time of day, with a
maximum variation of one hour more or less to avoid the effect of the circadian
cycle on blood pressure responses.
At
each visit, participants were tested in random order under one of the four
experimental treatments. The subjects were submitted to different percentages
of AOP: 30%, 50%, 80%, and 100% (30BFR, 50BFR, 80BFR, and 100BFR,
respectively).
Upon
reaching the laboratory, the volunteers rested on a stretcher in the supine
position for 10 minutes. At the end of this period, the pressure level
representing the AOP was identified using ultrasound equipment in Doppler mode.
This procedure took between 40 to 60 seconds. After 20 minutes of recovery in
the supine position, the subjects were submitted, in random order, to one of
the experimental treatments, in order to have the hemodynamic variables
monitored for five minutes, as follows: one minute to obtain baseline
measurements, two minutes with the cuff inflated in the proximal portion of the
right upper limb and two minutes of observation with the cuff deflated. The
NIRS measurements O2Hb, HHb, tHb, and TSI, were collected continuously during the five
min test procedure. Figure 1 shows the procedures performed.
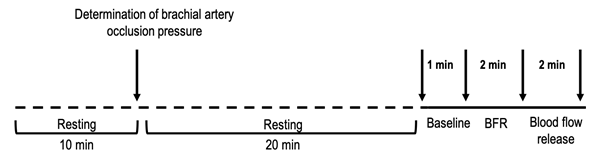
Caption
= BFR - blood flow restriction; the arrows indicate the start and/or end of
each phase (Baseline, BFR and flow released)
Figure 1 - Experimental
design. Treatment conditions (30%, 50%, 80% and 100% of brachial artery blood
flow occlusion pressure) were randomly assigned for each subject
Determination of
brachial artery blood flow occlusion pressure (AOP)
The
AOP was determined with an ultrasound scanner (Logic e, General Electric - GE
Healthcare, Milwaukee, WI, USA) equipped with Doppler. A 10 cm-wide cuff was
positioned at the most proximal portion of the right arm. A 40 mm-ultrasound
transducer was placed on the anteromedial face of the right arm. The transducer
was positioned perpendicular to its axis, 5 to 10 cm above the antecubital
fold. The cuff pressure was progressively slowly released until the first sign
of flow was observed in the brachial artery procedure was repeated two or three
more times to confirm the pressure level of the cuff, operationally defined as
the AOP. This procedure was performed in all visits and lasted approximately
one minute.
Near infrared
spectroscopy (NIRS) measurements
Monitoring
of the muscle hemodynamics was performed using near-infrared spectroscopy
(NIRS). This is a non-invasive optical technique that measures changes in the
relative concentration of oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb) in arterioles, venules, and capillaries [19]. The
electrons of the hemoglobin chromophores can absorb light near the infrared
region at different peaks according to the presence or absence of oxygen bound
to the hemoglobin molecule [21]. In this way, using the Lambert-Beer law, it is
possible to calculate changes in the concentration of the chromophores of
interest, such as O2Hb or HHb. Changes in
the concentrations of oxygenated hemoglobin (O2Hb), deoxygenated (HHb), total hemoglobin (tHb = O2Hb
+ HHb), and the tissue saturation index (TSI) were
measured continuously for 5 min in all experimental
conditions (30BFR, 50BFR, 80BFR and 100BFR), using a near-infrared
continuous-wave spectrometer (NIRS; PortaMon, Artinis Medical Systems BV, Zetten,
Netherlands). The total hemoglobin concentration (tHb)
was obtained by adding the concentration of O2Hb with HHb and is an indirect indicator of blood volume. The TSI
is a direct indication of the percentage of oxygenated hemoglobin and was
obtained through the following equation: TSI (%) = (O2Hb/tHb) x 100.
The
sensor was positioned in the most distal position on the belly of the biceps
brachii muscle. The sensor was surrounded by a plastic film, attached to the skin
by tape, and covered with a dark towel to avoid distortion of the signal caused
by sweat and ambient light. Data were collected using dedicated OxySoft software version (OxySoft
Ver. 2.1.1-2.1.6 Artinis Medical Systems BV, Zetten, Netherlands) with a sampling frequency of 10 Hz.
Statistical
analysis
NIRS
variables values at baseline were obtained by averaging the 15 s before blood
flow restriction. Measurements were obtained at the end of a 2-min period of
blood flow restriction and 30 s after deflation of the cuff. All measurements
obtained during and after blood flow restriction were normalized by the
baseline obtained on the same day to reduce the influence of the measurements
collected on different days.
After
testing the assumptions of normality and sphericity using Shapiro-Wilk and
Mauchly tests, respectively, a two-way ANOVA with repeated measures was used to
determine a significant interaction difference between treatments and time
conditions. Where significant F was observed, Sidak’s
post hoc test was applied to analyze possible differences in the dependent
variables among conditions (30BFR, 50BFR, 80BFR, and 100BFR) within each phase
(BFR and blood flow release). The level of significance adopted in this study
was 0.05. Also, the effect size (ES) was used to identify the clinical effect
through the magnitude of the difference [22,23].
Except
for the variable O2Hb in groups 30BFR, 50BFR, and 100BFR during the
blood flow restriction phase, TSI in group 30BFR, and tHB
in 100BFR, all other variables showed normal distribution. However, the ANOVA
test was used in all analyses because it is robust enough to be used even when
normality is not observed [24]. The Greenhouse-Geisser
correction was used to compare TSI between the conditions due to the violation
of sphericity.
All
analysis were performed using commercially available
SPSS statistical software (IBM Corp. Released 2016. IBM SPSS Statistics for
Windows, Version 21.0. Armonk, NY: IBM Corp).
Results
Post-hoc
analysis identified the study's power at 0.83. For this result, an effect size
of 0.40 was considered, an error α: 0.05, for a sample size of 10
participants, in four conditions (of blood flow restriction), three measures
repeated over time (baseline, restriction of blood flow and after the release
of arterial flow), a correlation between repeated measures of 0.8 and
non-sphericity correction of 1.
After
the blood flow was released, a significant difference was observed in TSI
between the 30BFR and 50BFR (p = 0.012) and 30BFR and 100BFR (p = 0.006). The tHb showed significant difference between the 30BFR and
50BRF (p = 0.037), between 50BFR and 100BFR (p = 0.002), and between 80BFR and
100BFR (p = 0.007). In addition, a significant difference was observed in O2Hb
between the 30BRF and 100BFR (p = 0.000), 50BFR and 100BFR (p = 0.000) and
between 80BFR and 100BFR (p = 0.007) conditions. Finally, HHb
showed significant difference between 30BFR and 50BFR (p = 0.032), as well as
between 30BRF and 80BFR (p = 0.007). Figure 2 shows the results of each
dependent variable evaluated in this study. For comparisons within groups,
there was a difference in O2Hb between all conditions (baseline,
blood flow restriction, and blood flow released), except for 100BFR (baseline
vs. blood flow restriction; p = 0.999) and 30BFR (baseline vs. blood flow
released; p = 0.699). For tHB there was a difference
for all combinations, except 50BFR (p = 0.991) and 80BFR (p = 0.995) between
baseline and blood flow released. In HHb conditions,
only there was no difference between baseline and blood flow released to 30BFR
(p = 0.258) and 100BFR (p =0.225). Finally, there was a significant difference
in all conditions over time to TSI.
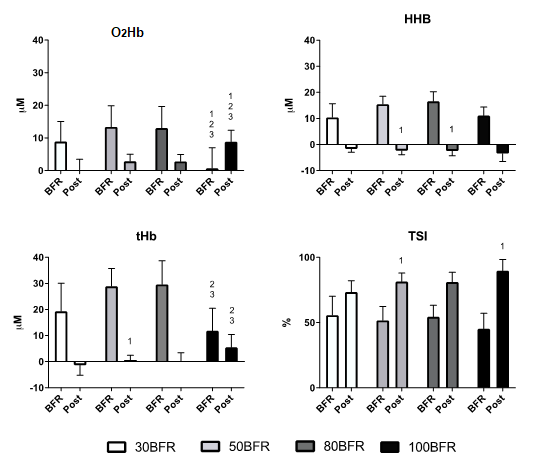
All
values during BFR were statistically different from baseline in each pressure
level. All values post-BFR were statistically different from during BFR in each
pressure level. 1 = different from 30BFR; 2 = different from 50BFR; 3 =
different from 80BFR. All differences for p < 0.05
Figure 2 - Oxyhemoglobin
(O2Hb), desoxyhemoglobin (HHb), total hemoglobin (tHB) and
tissue saturation index (TSI) modifications from baseline at the different body
flow restriction pressure levels (30%, 50% 80% and 100% BFR) during blood flow
restriction (BFR) and after flow release (Post)
The
effect size varied from very small to huge in the most diverse combinations
between groups, according to Sawilowsky's
classification [25]. The following Tables I to III show the results of all
effect sizes related to TSI, tHb, O2Hb,
and HHb. In Table I, it was possible to observe that
the most significant clinical impacts between the measurements obtained during
and after blood flow restriction occurred in the TSI and HHb
measurements in all restriction conditions. In the tHB
variable, the 100BFR condition had the lowest clinical impact, while in the O2Hb
variable, the 50BFR and 80BFR conditions had a huge effect.
Table
II shows the clinical impact of the difference between groups during the period
of blood flow restriction. The 100BFR condition had larger effect sizes than
all other conditions for the variables. O2Hb, tHb,
and TSI, indicating that this condition is the one that generated the most
significant impact on tHb and muscle oxygenation
while the cuff was inflated. On the other hand, the impact of changes in tHb and muscle oxygenation between 30BFR and 50BFR were the
smallest.
Table I - Effect
size (ES) of the dependent variables TSI, O2Hb, HHb
e tHb for repeated measures (BFR vs. Post-BFR)
between treatments, based on the criteria proposed by Sawilowsky
[25]
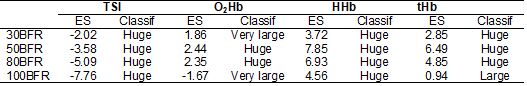
TSI
= Tissue Saturation Index; O2Hb = Oxyhemoglobin; HHb
= Deoxyhemoglobin; tHb = Total Hemoglobin; Classif: classification
Table II - Effect
size (ES) of the dependent variables TSI, O2Hb, HHb
and tHb for treatment comparisons during blood flow
restriction, based on the criteria proposed by Sawilowsky
[25]
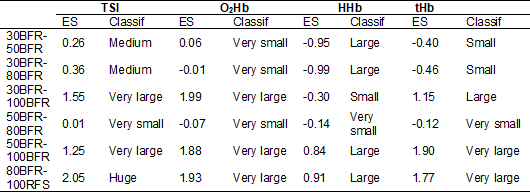
TSI
= Tissue Saturation Index; O2Hb = Oxyhemoglobin; HHb
= Deoxyhemoglobin; tHb = Total Hemoglobin; Classif: classification
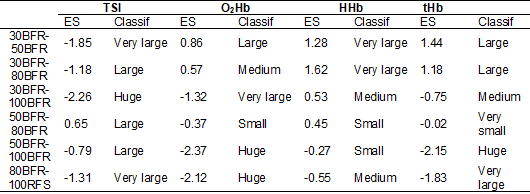
Table
III shows the clinical impact of the difference between treatments after the
period of blood flow restriction. All conditions of blood flow restrictions
showed a large effect size between the TSI variable, indicating that each
change in the restriction range causes a great clinical impact on muscle
oxygenation. Muscle volume measured indirectly by tHb
indicated a little clinical impact on the change observed between 50BFR and
80BFR, but the other changes at each restriction range change occurred with
greater impact.
Table III - Effect size (ES) of the dependent variables TSI, O2Hb, HHb and tHb for condition
comparisons, after blood flow release, based on the criteria proposed by Sawilowsky [25]
TSI
= Tissue Saturation Index; O2Hb = Oxyhemoglobin; HHb
= Deoxyhemoglobin; tHb = Total Hemoglobin; Classif: classification
Discussion
This
study showed that different levels of blood flow restriction in the upper limb
do not promote linear changes in the percentage of tissue oxygenation and total
hemoglobin. This finding agrees with previous studies that also identified that
total hemoglobin reduction is not linear with pressure load.
The
differential of the current study was, in addition to observing the behavior of
total hemoglobin (indirectly), having followed the impact on cellular hypoxia.
Identifying non-linear behavior in cellular hypoxia is important because this
seems to be a stimulus condition for muscle hypertrophy mechanisms [18].
The
current results demonstrate that it is unnecessary to exert
high-pressure
loads to significant impact hypoxia, allowing the participant to
reach a possible hypertrophic stimulating condition with loads between
30 and 50% of total occlusion, without experiencing the significant
discomfort generated
by higher loads.
The
main findings were as follows. Tissue saturation index (TSI) decreased under
all conditions, significant for 100BFR compared to 80BFR during the blood flow
restriction phase. After the release of blood flow, a significant increase was
observed in all conditions, indicating a rebound effect, and for loads of
100BFR and 50BFR, those showed more significant effect than 30BFR. The tHb value is higher in moderate blood flow restriction
loads, mainly 50BFR and 80BFR compared to more extreme pressure level (100BFR).
The oxygenated hemoglobin increased significantly with submaximal pressure
load. However, after the release of blood flow, O2Hb augmented for
100BFR conditions while the other groups decreased. Furthermore, the muscle
oxygenation returned to baseline condition for 30BFR. Finally, the deoxygenated
hemoglobin was higher in medium and high blood pressure loads (50BFR and 80BFR)
when compared to lower blood flow restriction loads (30BFR) after the release
of the blood flow.
TSI
is a direct indicator of the percentage of oxygenated hemoglobin in the tissue
directly below the sensor. The present study observed a reduction in TSI
concentration during inflated cuff, which indicates that the oxygenated blood
supply is less than muscle demand. The uptake of muscle oxygen can be
influenced, among other factors, by the ability of the microcirculation to
provide the necessary oxygen to the tissue [19]. The reduction in TSI has
already been observed in other studies of blood flow restriction associated or
not with the practice of physical exercise [25,26].
Kilgas et al. [20] observed a significant reduction
in TSI compared to the control condition in four different pressure loads (60%,
80%, 100%, and 120% of the AOP) associated with the handgrip exercise. The
authors identified a more significant reduction in TSI as the pressure level
increased, but with no difference between 60 and 80% (submaximal loads) and
between 100 and 120% of the AOP (maximum and supra-maximum, respectively).
Although the present study did not associate blood flow restriction with
exercise, there was also a tendency to reduce the TSI as the pressure level
increased, with no significant difference between submaximal loads
(baseline > 30BFR = 50BFR = 80BFR > 100BFR). Both studies used a 10 cm
wide cuff.
A
hyperemic rebound effect allowed the TSI indicators to remain higher than the
resting condition even after 30 seconds of withdrawal of the cuff pressure.
This result is reinforced by the clinical difference observed through the
effect size obtained in the multiple comparisons between the conditions in the
present study. The effect size was considered very small to medium between the
30BFR, 50BFR, and 80BFR conditions, but very large to huge when these
intermediates were compared to 100BFR. Thus, the 100BFR had a more significant
impact on the TSI compared to the other conditions.
In
practical terms, the similar lower oxygen saturation between the 30BFR, 50BFR,
and 80BFR experimental treatments indicates that this flow restriction margin
appears to have a similar impact on cellular hypoxia. Disregarding exercise, a
restriction between 30 and 80% of the AOP could have a similar impact on the
hypertrophic mechanisms associated with the more metabolic environment.
Previous studies have shown that simple exposure to blood flow restriction
without exercise can promote hypertrophic stimuli that would reduce the impact
of atrophy caused by an injury to the muscle-tendon structure [13]. Thus, the
lower pressure level (30%) may be more comfortable and safer for most people,
particularly older and untrained individuals, providing similar benefits to an
80% arterial pressure level restriction. On the other hand, higher pressures
that allow total or close to AOP would probably promote a higher hypoxic
ambient despite being more uncomfortable. Such a more favorable milieu would
potentiate mechanisms such as cell swelling [10] and the release of growth
hormone (GH) [11].
Although
Hunt et al. [27] have observed that the deformation of the brachial
artery occurs at approximately 110 mmHg of pressure with the use of an 11 cm
wide cuff, the reduction in blood flow occurs early in order to change the
arterial diameter. On average, men experienced a reduction in blood flow with
60 mmHg.
In
another study by Mouser et al. [17], the venous system was impacted with
pressure loads of 10 to 30% of AOP. Notwithstanding, the artery would only be
impacted with pressure loads higher than 60%. This study was carried out with a
5 cm cuff, half the width of the cuff in the present study. The literature has
shown that cuffs with a smaller width require a higher-pressure level to cause
a similar impact to a broader cuff [28].
The
present study observed an increase in tHB in all
pressures used in relation to the baseline and being more significant in the
conditions 50BFR and 80BFR compared to 100BFR (baseline> 30 BFR = 100BFR
> 50BFR = 80BFR). When considering the results of these previous studies
with the current observations, the 50BFR condition was performed with an
average pressure level of 68 mmHg. It is possible to assume that 50BFR and
80BFR must have interfered equally in the blood flow, as noted by the small
effect size between these conditions. This assumption is supported by another
study by Mouser et al. [16]. The authors identified blood flow reduction
up to 50% of arterial occlusion, followed by stabilizing the flow up to 90%
before another sudden drop. This abrupt reduction in blood flow in the last 10%
before reaching the point of AOP should explain why 100BFR had a lower tHb than intermediate pressure loads (50BFR and 80BFR).
After the cuff deflated, blood flow observed by the tHb
concentration returned to rest at 50BFR and 80BFR, but not at 30BFR and 100BFR.
The
concentration of HHb is an important indicator of
oxidative metabolism in muscles [29]. The increase in the concentration of HHb in all restriction conditions performed in the present
study indicates the hypoxia generated by the mechanical restriction. This
result agrees with what was observed in a previous study [30]. Although there
was no difference between groups during the restriction phase, the effect size
indicated that pressure levels between 50BFR and 80BFR had a more significant
clinical impact concerning all conditions and with very little practical
difference between them. Furthermore, all groups had a substantial clinical
difference and statistically significantly higher HHb
values than baseline.
The
significance of this finding is that hypoxia is an important signal to
stimulate some mechanisms of strength and muscle mass increase [11,31]. Thus,
it is possible to assume that, at least at rest, the 30BFR level has the same
impact on hypoxia and muscle oxidative metabolism as on higher pressure levels,
thus reducing discomfort and cardiovascular risk. On the other hand, after
blood flow release, HHb values reduced in all groups,
but only at 30BFR and 100BFR did the levels return to baseline condition after
30 seconds.
Finally,
a more pronounced increase in O2Hb levels in the 30BFR, 50BFR, and
80BFR than the 100BFR condition was observed during the cuff inflation phase.
The very high clinical impact between the 100BFR and the other pressure levels
confirmed this distinct behavior between maximum and submaximal pressure loads.
On the other hand, observing a minimal effect size between submaximal loads
demonstrated that the clinical implications generated with an arterial
restriction level of 30 to 80% of the AOP at rest are practically
insignificant.
The O2Hb
concentration during blood flow restriction in the 100BFR condition was similar
to the baseline. Besides, a reduction in O2Hb concentrations in the
30BFR, 50BFR, and 80BFR conditions was observed when the cuff pressure was
released. Nevertheless, these values did not return to the baseline condition
within 30 seconds of free blood flow. On the other hand, in the 100BFR
condition, the O2Hb concentration increased, indicating a possible
rebound effect due to the action of some vasodilating substances, such as
nitric oxide [32]. After the flow is released, blood moves more turbulently,
increasing shear stress stimulating the production and release of nitric oxide,
promoting local vasodilation [33,34]. Shear stress is influenced by blood flow
speed, which is altered according to the pressure imposed by the cuff and blood
flow release by removing the pressure exerted by the cuff [35]. A higher
concentration of O2Hb accompanies this increase in blood flow.
These results are opposite with those
observed in previous studies. Such studies observed a reduction in O2Hb
during blood flow restriction [36], possibly due to the difference in the site
of signal capture between the studies. The difference observed in the results
during the blood restriction phase may be explained by the positioning of the
NIRS probe about the site of compression exerted by the cuff. Bopp et al.
[36] positioned the probe on the subject's forearm immediately after the cuff,
restricting the blood, while in the present study, the NIRS probe was placed on
the arm, and the cuff was placed on the forearm. This procedure was done to
avoid interference in the vascular walls due to deformation by the inflated
cuff.
The
NIRS device captures hemoglobin concentrations (oxy and deoxygeated)
to a depth of 1.5 cm below the transmitter/receiver. Thus, the relative O2Hb
concentration is measured in the small blood vessels (arterioles, venules, and
capillaries) that cross this region captured by the equipment. In the 100BFR
condition, the blood flow must have been interrupted or close to it, even in
the deepest regions, and it must have kept the O2Hb concentration in
the arteries located before the inflated cuff. The equipment should not have
picked up the blood in the most profound vessels.
Some
limitations do apply to the present study. We implemented BFR during rest, and
different muscle hemodynamic behaviors may be expected during resistance
exercise. Besides, our findings are limited to the upper limbs and may not
entirely represent blood flow restriction involving a larger muscle mass.
Therefore, further studies are needed to confirm a possible relationship
between acute muscle hemodynamics caused by different blood flow restriction
pressure levels and hypertrophic markers secondary to resistance exercise with
blood flow restriction.
Conclusion
In
conclusion, this study revealed that pressure levels between 30 and 50%
of the brachial artery blood flow occlusion are sufficient to cause
hypoxia in the occluded muscles, in the sama magnitude as higher
pressure loads (up to 80%). That pressure range appears
to represent a comfortable and sufficient stimulus to increase muscle
metabolism and metabolic stress associated with hypertrophy induced by
the
resistance training with blood flow restriction.
Conflict
of interest
All
authors declare that there is no conflict of interest regarding this study and
manuscript
Funding
source at the time of the study
Gomes
PS - Productivity in Research Scholarship (PQ2) from the National Council for
Scientific and Technological Development from Brazil - CNPq;
PROCIÊNCIA Scholarship sponsored by the State University of Rio de Janeiro
Carvalho
RF - PhD scholarship holder from the Carlos Chagas Filho Foundation for
Research in the State of Rio de Janeiro - FAPERJ (Proc.: E-26/201.705/2017)
Author´s
contributions
Conception
of the study: Gomes PSC, Meirelles CM; Study design: Carvalho RF,
Gomes PSC, Fernandes Junior ML, Meirelles CM; Data
collection: Carvalho RF, Fernandes Junior ML; Statistical
analysis: Carvalho RF, Gomes PSC; Writing of the document: Carvalho
RF, Gomes PSC, Fernandes Junior ML, Meirelles CM; Final
review of the manuscript: Meirelles CM; Writing
the English version of the manuscript: Gomes PSC
References
- Lixandrão ME, Ugrinowitsch
C, Laurentino G, Libardi CA, Aihara
AY, Cardoso FN, et al. Effects of exercise intensity and occlusion pressure
after 12 weeks of resistance training with blood-flow restriction. Eur J
Appl Physiol 2015;115(12):2471-80. doi: 10.1007/s00421-015-3253-2 [Crossref]
- Lixandrão ME, Ugrinowitsch C, Berton R, Vechin FC, Conceição MS, Damas F, et al. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med 2018;48(2):361-78. doi: 10.1007/s40279-017-0795-y [Crossref]
- Martín-Hernández
J, Marín PJ, Menéndez H, Ferrero C, Loenneke JP,
Herrero AJ. Muscular adaptations after two different volumes of blood
flow-restricted training. Scand J Med Sci Sports
2013;23(2):1-7. doi: 10.1111/sms.12036 [Crossref]
- Vechin FC, Libardi CA, Conceição MS, Damas
FR, Lixandrão ME, Berton
RPB, et al. Comparisions between low-intensity
resistance training with blood flow restriction and high-intensity resistance
training on quadriceps muscle mass and strength in elderly. J Strength Cond Res
2015;29(4):1071-6. doi: 10.1088/0022-3727/8/4/003 [Crossref]
- Yasuda T, Ogasawara R, Sakamaki M, Ozaki H, Sato Y, Abe T. Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur J Appl Physiol 2011;111(10):2525-33. doi: 10.1007/s00421-011-1873-8 [Crossref]
- Takarada Y, Nakamura Y, Aruga S, Onda T, Miyazaki S, Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J Appl Physiol. 2000;88(1):61-5. doi: 10.1152/jappl.2000.88.1.61 [Crossref]
- Takarada Y,
Sato Y, Ishii N. Effects of resistance exercise combined with vascular
occlusion on muscle function in athletes. Eur J Appl Physiol
2002;86(4):308-14. doi: 10.1007/s00421-001-0561-5 [Crossref]
- Takarada Y, Tsuruta T, Ishii N. Cooperative effects of exercise and occlusive stimuli on muscular function in low-intensity resistance exercise with moderate vascular occlusion. Jpn J Physiol 2004;54(6):585-92. doi: 10.2170/jjphysiol.54.585 [Crossref]
- Laurentino GC, Ugrinowitsch C, Roschel H, Aoki MS, Soares AG, Neves Junior M, et al. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc 2012;44(3):406-12. doi: 10.1249/MSS.0b013e318233b4bc [Crossref]
- Loenneke JP, Fahs CA, Rossow LM, Abe T, Bemben MG. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses 2012;78(1):151-4. doi: 10.1016/j.mehy.2011.10.014 [Crossref]
- Reeves GV, Kraemer RR, Hollander DB, Clavier J, Thomas C, Francois M, et al. Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. J Appl Physiol 2006;101:1616-22. doi: 10.1152/japplphysiol.00440.2006 [Crossref]
- Lopes KG, Bottino DA, Farinatti P, Souza MGC, Maranhão PA, Araujo CMS, et al. Strength training with blood flow restriction – a novel therapeutic approach for older adults with sarcopenia? A case report. Clin Interv Aging 2019;14:1461-9. doi: 10.2147/CIA.S206522 [Crossref]
- Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc 2000;32(12):2035-9. doi: 10.1097/00005768-200012000-00011 [Crossref]
- Loenneke JP, Thiebaud RS, Abe T, Bemben MG. Blood flow restriction pressure recommendations: The hormesis hypothesis. Med Hypotheses 2014;82(5):623-6. doi: 10.1016/j.mehy.2014.02.023 [Crossref]
- Mattocks KT, Jessee MB, Counts BR, Buckner SL, Mouser JG, Dankel SJ, et al. The effects of upper body exercise across different levels of blood flow restriction on arterial occlusion pressure and perceptual responses. Physiol Behav 2017;171:181-6. doi: 10.1016/j.physbeh.2017.01.015 [Crossref]
- Mouser JG, Dankel SJ, Jessee MB, Mattocks KT, Buckner SL, Counts BR, et al. A tale of three cuffs: the hemodynamics of blood flow restriction. Eur J Appl Physiol 2017;117(7):1493-9. doi: 10.1093/icvts/ivx022 [Crossref]
- Mouser JGACJ, Black CD, Bemben DA, Bemben MG. Brachial blood flow under relative levels of blood flow restriction is decreased in a nonlinear fashion. Clin Physiol Funct Imaging 2018;38(3):425-30. doi: 10.1111/cpf.12432 [Crossref]
- Schoenfeld BJ.
Potential mechanisms for a role of metabolic stress in hypertrophic adaptations
to resistance training. Sports Med 2013;43(3):179-94. doi: 10.1007/s40279-013-0017-1 [Crossref]
- Gerovasili V, Dimopoulos S, Tzanis G, Anastasiou-Nana M, Nanas S. Utilizing the vascular
occlusion technique with NIRS technology. Int J Ind Ergon 2010;40(2):218-22. doi: 10.1016/j.ergon.2009.02.004 [Crossref]
- Kilgas MA,
McDaniel J, Straves J, Pollock BS, Singer TJ, Elmer
SJ. Limb blood flow and tissue perfusion during exercise with blood flow
restriction. Eur J Appl Physiol 2019;119(2):377-87. doi: 10.1007/s00421-018-4029-2 [Crossref]
- Pereira MIR, Gomes, PSC, Bhambhani,
YN. A brief review of the use of near infrared spectroscopy with particular
interest in resistance exercise. Sports
Med 2007;37:615-24. doi: 10.2165/00007256-200737070-00005 [Crossref]
- Espírito-Santo HA, Daniel F. Calcular e apresentar tamanhos do efeito em trabalhos científicos (1): As limitações do p < 0,05 na análise de diferenças de médias de dois grupos. Rev Port Inv Comp Soc 2015;1(1):3-16. doi: 10.7342/ismt.rpics.2015.1.1.14 [Crossref]
- Espírito-Santo HA, Daniel F. Calcular e apresentar tamanhos
do efeito em trabalhos científicos (3): Guia para reportar os tamanhos do
efeito para análises de regressão e ANOVAs
Calculating and reporting effect sizes on scientific
papers (3): Guide to report regression
models and ANOVA. Rev
Port Inv Comp Soc 2018;4(1):43-60. doi: 10.7342/ismt.rpics.2018.4.1.72 [Crossref]
- Vincent WJ, Weir
JP. Statistics in Kinesiology. 4th edition ed. [S. l.]: Human Kinetics, Inc.,
2011. E-book
- Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods 2009;8(2):article26. doi: 10.22237/jmasm/1257035100 [Crossref]
- Padilla J,
Johnson BD, Newcomer SC, Wilhite DP, Mickleborough
TD, Fly AD, et al. Normalization of flow-mediated dilation to shear stress area
under the curve eliminates the impact of variable hyperemic stimulus.
Cardiovasc Ultrasound 2008;6(1):44. doi: 10.1186/1476-7120-6-44 [Crossref]
- Hunt JEA, Stodart C, Ferguson RA. The influence of participant characteristics on the relationship between cuff pressure and level of blood flow restriction. Eur J Appl Physiol 2016;116(7):1421-32. doi: 10.1007/s00421-016-3399-6 [Crossref]
- Crenshaw AG, Hargens AR, Gershuni DH, Rydevik B. Wide tourniquet cuffs more effective at lower
inflation pressures. Acta Orthop Scand
1988;59(4):447-51. doi: 10.3109/17453678809149401 [Crossref]
- Ryan TE, Brophy P, Lin C, Hickner RC, Neufer PD. Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol 2014;592(15):3231-41. doi: 10.1113/jphysiol.2014.274456 [Crossref]
- Soares RN, McLay KM, George MA, Murias JM. Differences in oxidative metabolism modulation induced by ischemia/reperfusion between trained and untrained individuals assessed by NIRS. Physiol Reports 2017;5(19):1-7. doi: 10.14814/phy2.13384 [Crossref]
- Moritani T, Sherman WM, Shibata M, Matsumoto T, Shinohara M. Oxygen availability and motor unit activity in humans. Eur J Appl Physiol and Occup Physiol 1992;64(6):552-6. doi: 10.1007/BF00843767 [Crossref]
- Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thiissen DHJ. Is flow-mediated dilation nitric oxide mediated? A meta-analysis. Hypertension 2014;63(2):376-82. doi: 10.1161/HYPERTENSIONAHA.113.02044 [Crossref]
- Doshi SN, Naka
KK, Payne N, Jones CJH, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated
dilatation following wrist and upper arm occlusion in humans: the contribution
of nitric oxide. Clin Sci
2001;101(6):629-35. doi: 10.1042/cs1010629 [Crossref]
- Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ
Alexander RW, et al. Regulation of endothelial cell nitric oxide synthase
mRNA expression by shear stress. Am J Physiol - Cell Physiol 1995;269(6):38-46. doi: 10.1152/ajpcell.1995.269.6.c1371 [Crossref]
- Gnasso A, Carallo C, Irace C, Franceschi MS, Mattioli PL, Motti C, Cortese C. Association between wall shear stress and flow-mediated vasodilation in healthy men. Atherosclerosis 2001;156(1):171-6. doi: 10.1016/S0021-9150(00)00617-1 [Crossref]
- Bopp CM,
Townsend DK, Barstow TJ. Characterizing near-infrared spectroscopy responses to
forearm post-occlusive reactive hyperemia in healthy subjects. Eur J Appl Physiol 2011;111(11):2753-61. doi: 10.1007/s00421-011-1898 [Crossref]
