Rev Bras Fisiol Exerc 2022;21(1):48-60
doi: 10.33233/rbfex.v21i1.4982ORIGINAL ARTICLE
Acute effects of blood flow restriction resistance exercise on
endothelial function and platelet aggregation
Efeitos agudos do
exercício contrarresistência com restrição de fluxo
sanguíneo na função endotelial e agregação plaquetária
Claudia Mello Meirelles1,
Marcio Lopes Fernandes Júnior2, Cristiane Matsuura3,
Paulo Sergio Chagas Gomes3
1Escola de Educação Física do Exército,
Rio de Janeiro, RJ, Brazil
2Universidade Estácio de Sá, Rio de
Janeiro, RJ, Brasil
3Universidade do Estado do Rio de
Janeiro, RJ, Brazil
Received: November 13,
2021; Accepted: February 9,
2022.
Correspondence: Paulo Sergio Chagas Gomes, Ph.D.,
Universidade do Estado do Rio de Janeiro, Instituto de Educação Física e
Desportos, Rua São Francisco Xavier, 524
Claudia
Mello Meirelles: claudiameirelles@yahoo.com.br
Marcio
Lopes Fernandes Júnior: marcio.eefd@gmail.com
Cristiane Matsuura: crismatsuura@gmail.com
Paulo
Sergio Chagas Gomes: paulo.gomes@uerj.br
Abstract
Objective: To compare endothelial function and platelet
aggregation after resistance exercise performed with low-intensity blood flow
restriction (LI-BFR) or free blood flow (LI-FreeBF)
and high intensity with no blood flow restriction (HI-FreeBF)
in healthy adults. Methods: Ten healthy men (23 ± 3 years) performed
three experimental trials involving bilateral leg press and knee extension in a
randomized crossover design: LI-BFR (3 x 15 reps at 30% 1 RM), LI-FreeBF (3 x 15 reps at 30% 1 RM), and HI-FreeBF (3 x 8 reps at 80% 1RM). BFR was maintained at 50%
of the individual total occlusion pressure during the three sets, and it was
released after the end of set 3. Brachial artery flow-mediated dilation (FMD)
was measured with ultrasound with Doppler before and after exercise. Blood was
collected to determine nitrite levels and platelet aggregation. Results:
None of the volunteers reported any adverse reactions during the exercise
protocols. A 3 x 2 ANOVA with repeated measures in both factors (condition vs.
time) indicated no significant main effects or interactions for FMD, basal and
peak brachial artery diameter, and shear rate. Plasma nitrite levels and
platelet aggregation did not differ among the three exercise conditions nor
pre-post resistance exercise. Conclusion: Our results indicate that
lower limbs resistance exercise performed at low or high intensities and with
or without BFR does not affect endothelial function, nitrite levels, and
platelet aggregation. These findings indicate that such exercise conditions do
not seem to represent cardiovascular risk from a hemostatic point of view in
healthy adult men.
Keywords: oxyhemoglobin; deoxyhemoglobin; muscle strength; resistance
exercise; hemodynamics.
Resumo
Objetivo: Comparar a função endotelial e a
agregação plaquetária após exercícios contrarresistência
de baixa intensidade com restrição do fluxo sanguíneo (BI-RFS) ou fluxo
sanguíneo livre (BI-FSLivre) e alta intensidade com
fluxo sanguíneo livre (AI-FSLivre) em adultos
saudáveis. Métodos: Dez homens saudáveis (23 ± 3 anos) realizaram três
ensaios experimentais envolvendo leg press bilateral e extensão de joelho em um delineamento
cruzado randomizado: BI-RFS (3 x 15 repetições a 30% 1RM), BI- FSLivre (3 x 15 repetições a 30% 1RM) e AI-FSLivre (3 x 8 repetições a 80% 1RM). A RFS foi mantida em
50% da pressão de oclusão total individual durante as três séries, e foi
liberada após o final da série 3. A dilatação fluxo-mediada da artéria braquial
(DILA) foi medida com ultrassom com Doppler antes e após o exercício. O sangue
foi coletado para determinar as concentrações de nitrito e a agregação
plaquetária. Resultados: Nenhum dos voluntários relatou qualquer reação
adversa. A ANOVA 3 x 2 com medidas repetidas em ambos os fatores (condição vs.
tempo) não indicou efeitos principais ou interações significativas para DILA,
diâmetro basal e de diâmetro máximo da artéria braquial, nem força de
cisalhamento. Nitrito plasmático e agregação plaquetária não diferiram entre as
três condições de exercício nem pré-pós exercício. Conclusão:
O exercício contrarresistência de membros inferiores
realizado em baixa ou alta intensidade, com ou sem RFS não afeta a DILA, os
níveis de nitrito, nem a agregação plaquetária. Os achados indicam que tais
condições de exercício não parecem representar risco cardiovascular do ponto de
vista hemostático em adultos saudáveis.
Palavras-chave: oxiemoglobina; desoxiemoglobina;
força muscular; treinamento de força; hemodinâmica.
Introduction
Endothelial dysfunction is an important variable
involved in cardiovascular morbidities such as atherosclerosis, hypertension,
and coronary artery disease [1]. It has been shown that resistance exercise
(RE) can improve endothelial function in adults [2]. Nevertheless, despite
favorable long-term adaptations to training, a single RE bout can transiently
worsen endothelial function and increases platelet aggregation during and soon
after an individual takes part in an exercise session [3].
When associated with blood flow restriction,
resistance exercise (BFR-RE) has been recognized for its favorable effects on
strength and hypertrophy [4]. However, there is scarce evidence available on
their effects on vascular health.
Brachial artery flow-mediated dilatation (FMD) serves
as an index of nitric oxide (NO)-mediated endothelium-dependent vasodilator
function in humans and it is regarded as a surrogate marker of
vascular/endothelial function and cardiovascular disease [5].
Few studies have investigated the effects of
low-intensity BFR-RE on FMD. Evidence from chronic studies demonstrated
improvements [6], impairments [7], and no significant differences in FMD
following RE performed under free blood flow (FreeBF)
or BFR [8,9]. The only study investigating the acute effects of BFR-RE on
endothelial function [10] pointed to a decrease in FMD after a single bout of
handgrip exercise under BFR.
An acute increase in blood shear stress and the
inherent decrease in oxygen muscle saturation caused by BFR-RE may positively
affect endothelial health. Increased production of NO and activation of
vascular endothelial growth factor transcription [11,12] stimulates
angiogenesis and improves endothelial function. However, exercise can also
trigger factors with a negative impact, such as increased platelet aggregation
[13].
Studying the balance between
the vasoactive substances that favor vascular function and those that can
impair it is of paramount importance since an exercise session seems to be able
to activate both [10]. Given the scanty body of evidence on BFR-RE protocols
involving major muscle groups and real-world protocols, this study's objective
was to compare endothelial function and platelet aggregation after
low-intensity BFR-RE, low-intensity FreeBF, and
high-intensity resistance exercise in healthy adults.
Methods
Participants
Ten healthy male (23 ± 3 years) undergraduate physical
education students participated in this study. Subjects signed a written
informed consent form before the experimental procedures. The study was
conducted based on the ethical standards in Resolution 510/16 of the Brazilian
National Health Council, according to the recommendations defined in the
Declaration of Helsinki for research with human beings, signed at the 59th
Assembly of the World Medical Association in 2008. The institutional review
board at the State University of Rio de Janeiro approved the study protocol
(#3.125.780).
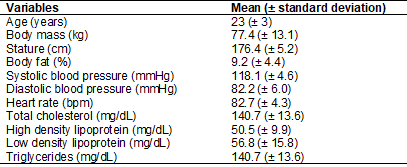
The morphological and health variables of the
participants are presented in Table I.
Study protocol
The study was conducted in a randomized and
crossover-controlled trial model. After the initial two visits, each subject
was randomly assigned to all three treatment conditions by drawing with no case
reposition.
Subjects reported to the laboratory on five occasions.
The first visit was used to explain the experimental procedures, take
anthropometric measures and collect data on pre-participation screening
(Physical Activity Readiness Questionnaire - Par-Q, Sheppard, 1988). On the
second visit, blood samples were drawn for characterizing the participants'
lipemic profile, and maximal dynamic strength tests (one-repetition maximum -
1RM) in the bilateral leg press and knee extension were recorded.
In the third, fourth, and fifth sessions, participants
underwent knee extension and leg press exercise routine under three
experimental conditions, in random order separated by three days of wash-out
period: 1) low intensity with restricted blood flow (LI-BFR); 2) low intensity
with free blood flow (LI-FreeBF), and 3) high
intensity with free blood flow (HI-FreeBF). Brachial
artery flow-mediated dilation (FMD) was measured by ultrasound before and after
exercise in each experimental condition. Blood samples were collected to
determine nitrite and platelet aggregation values.
The steps of the experimental procedures are shown in
Figure 1.
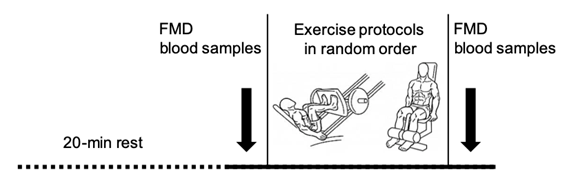
FMD = brachial artery flow-mediated dilation
Figure 1 - Experimental design
Determination of arterial occlusion pressure
A 17 cm x 68 cm wide nylon cuff (Tycos
Welch Allyn DS44-11) was used to identify the artery occlusion pressure (AOP).
The participants were asked to lie down in the prone position, with the cuff
applied to the proximal-most portion of the left thigh. Then, the 40 mm linear
array ultrasound probe (LOGIQe, GE Health Systems,
Minas Gerais, Brazil) was positioned over the popliteal artery in Doppler mode.
The cuff was continuously and slowly inflated until the pulse was silent. After
silence, the flow was slowly released to detect the pulse onset and inflated
again to silence to confirm AOP detection. The average AOP found was 152.0 ±
8.0 mmHg, and the average pressure applied throughout the exercise conditions
with BFR was 76.0 ± 4.1 mmHg.
Maximal dynamic strength tests
One-repetition maximum teste (1RM) was performed in
leg press and knee extension isotonic machines. Participants warmed up with an
estimated 50% of 1RM, using the following equation: 1RM = 100 x load / (102.78
– 2.78 x rep) (Nascimento et al., 2007). From the predicted value of 1
RM, the participants performed three to five attempts in an incremental
fashion, with intervals of 5 min, until the heaviest load that could be
successfully lifted once was determined.
The estimated 1 RM observed was 167.0 ± 17.9 kg for
leg press, and 89.0 ± 18.8 kg for knee extension.
Resistance exercise
All subjects were subjected to three experimental
conditions at random. The low-intensity condition with blood flow restriction
(LI-BFR) and free blood flow (LI-FreeBF) was
performed at 30% 1RM. The high-intensity condition with free blood flow (HI-FreeBF) was performed at 80% 1 RM in leg press and knee
extension exercises.
For the leg press, the applied load was 50.1 ± 5.4 kg
for LI-BFR and LI-FreeBF and 133.6 ± 14.3 kg for HI-FreeBF. For knee extension, the correspondent load was 26.7
± 5.7 kg and 71.2 ± 15.1 kg, respectively.
In the conditions LI-BFR and LI-FreeBF,
subjects performed three sets of 15 repetitions. In the HI-FreeBF
condition, the individuals performed three sets of eight repetitions, always
with a 1-min interval between sets in both exercises.
During BFR conditions, a 17 cm x 68 cm wide nylon cuff
(Tycos Welch Allyn DS44-11) was inflated and
maintained at 50% of the individual occlusion pressure. The cuff pressure was
released only after the completion of the third set.
Brachial artery hemodynamics
Brachial artery flow-mediated dilation (FMD) was
performed using a two-dimensional color spectral Doppler ultrasound equipped
with a 14-MHz linear transducer (Logic e, GE Health Systems, Brazil). Measures
were obtained before and immediately after each exercise condition, with the
subject lying in the supine position. The probe was placed on the right arm's
anteromedial face perpendicular to the forearm's centerline, 5-10 cm below the
antecubital fossa and over the artery. Basal and post-occlusion diameters were
continuously measured between the intima-lumen-intima interfaces. The occlusion
was maintained for 5 minutes using a 17 cm x 68 cm nylon cuff (Tycos Welch Allyn DS44-11) on the arm to apply pressure
slightly 50 mmHg above the systolic arterial pressure, which was confirmed by
the lack of a pulse on the Doppler screen. The procedure was recorded for a
total duration of 8 min: 1-min basal, 5-min of blood occlusion, and 2-min after
cuff deflation.
The same investigator performed all tests. FMD was
calculated as the percentage change in artery diameter after flow release (peak
diameter) about the basal diameter.
Brachial artery diameter and shear rate (4 times blood
velocity divided by diameter) were calculated: basal diameter and basal shear
rate corresponded to the average of the records obtained every second during
the first minute preceding the cuff inflation. Peak diameter and peak shear
rate were automatically detected as the highest values obtained during the
final two minutes of recording after cuff release. Off-line analyses of
diameters and shear rate were performed using automated edge-detection software
(Cardiovascular Suite, Quipu, Pisa, Italy).
Previous unpublished data from our laboratory showed a
high test-test reliability of FMD measurements, with intraclass correlation
coefficients of R = 0.83 and R = 0.78 for intraday and inter-day measurements,
respectively. The absolute typical error of measure was 0.8 % and 1.38 %.
Blood samples
A trained nurse performed all blood collections in
this study. Biochemical analyzes were performed by a blinded investigator, not
familiar with the testing procedures.
At baseline, blood samples (5 mL) were obtained by
venipuncture after a 12-hour fast. Levels of fasting total cholesterol,
high-density lipoprotein cholesterol, low-density-lipoprotein cholesterol, and
triglycerides were determined enzymatically with a Roche/Hitachi 917 system (A
F. Hoffmann-La Roche AG, Basel, Switzerland) and standard kits.
Nitric oxide production and platelet aggregation were
assayed before the basal FMD procedure and immediately after FMD was performed
post-exercise. A 5 mL sample of blood was drawn from the antecubital vein using
a sterile needle. For nitrite, blood samples were centrifuged at 4.000 rpm for
5 min to separate the plasma that was stored at -80 °C. The protein levels were
quantified by bicinchoninic acid assay (BCA kit, BioAgency,
Brazil), and the absorbance was read at 562 nm (TP-Reader, Thermoplate,
Brazil). The Griess assay assessed nitrite levels. The samples were mixed with
an equal volume (1:1) of Griess reagent (0.1% n-(1-naphthyl) ethylene-diamine
dihydrochloride, 1% sulfanilamide, and 2.5% H3PO4). The absorbance was measured
at 540 nm using a 96-well microplate reader (TP-Reader, Thermoplate,
Thermo Fisher, Waltham, MA, USA).
The platelet aggregation analysis was performed within
2 hours after blood collection. Firstly, platelet-rich plasma was obtained by
centrifugation at 200 g for 15 min. Subsequently, the platelet-poor plasma was
obtained by centrifugation at 900 g for 10 minutes, according to the method of
Yun-Choi et al. (2000). Platelet count was adjusted to 150.000 cells/µL
with platelet poor plasma. Platelet aggregation was quantified according to Born's technique (1962) in an optical aggregometer (Aggro/Link Model 810-CA. Chrono-Log, EUA) at a temperature
of 37o C, using ADP as agonist (ADP at concentrations of 5.0 uM).
Statistical analysis
All data are expressed as mean and standard deviation.
Shapiro-Wilk normality test for small samples was used to verify data departure
form normality. A two-way ANOVA with repeated measures on two factors (3 x 2,
condition vs. time) was used to identify differences in FMD and blood variables
between LI-FreeBF, LI-BFR, and HI-FreeBF
conditions. The Bonferroni post-hoc test was used to detect specific
differences when a significant main effect was found. All analyses were
performed using a commercial software (IBM SPSS Statistics for Windows, Version
21.0. Armonk, NY: IBM Corp). The level of statistical significance was set at a
P-value < 0.05.
The power of the test was determined based on the
F-test statistics family, using an ANOVA with repeated measures and
within-between factors interaction. Post-hoc analysis identified the study's
power at 0.74. For this result, an effect size of 0.40 was considered, an error
α: 0.05, for a sample size of 9 participants (there was one sample loss
for platelet aggregation and other sample loss to nitrite), in three conditions
(LI-FreeBF, LI-BFR and HI-FreeBF),
two measures repeated over time (pré vs post
treatment), a correlation between repeated measures of 0.8 and non-sphericity
correction of 1.
Results
Participants characteristics
None of the volunteers reported any adverse reactions
during the exercise protocols. All participants negatively answered the PAR-Q
questionnaire.
All variables tested did not show any statistical
significance departure from normality.
Table I - Participants
characteristics
Brachial artery hemodynamics
Ultrasound data of sufficient quality were not
collected for three participants, and their data were excluded from the final
hemodynamic analysis.
Results from 3 x 2 ANOVA for
basal and peak brachial artery diameter (BAD), basal and peak shear rate (SR)
(Table II) measured before and after the three exercise protocols indicated
that there were no significant main effects or condition vs. time interactions.
No significant differences for main effects or condition vs. time interaction
were found for FMD (Figure 2).
Table II - Brachial artery hemodynamics responses to the
three experimental resistance exercise conditions
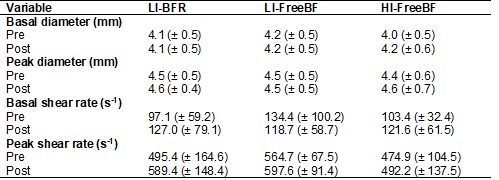
Data are reported as means ± SD for seven
participants; LI-BFR = low-intensity blood flow resistance exercise; LI-FreeBF = low-intensity resistance exercise with free blood
flow; HI-FreeBF = high-intensity resistance exercise
with free blood flow
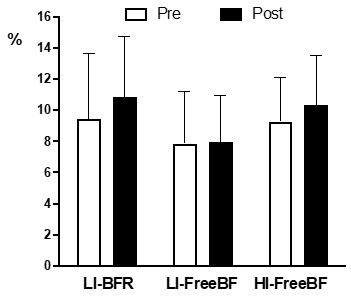
LI-BFR = low intensity with restricted blood flow; LI-FreeBF = low intensity with free blood flow; HI-FreeBF = high intensity with free blood flow; mean and
standard deviation. No differences were statistically significant for P <
0.05
Figure 2 - Brachial artery flow-mediated dilation measured
at pre- and post-exercise under different experimental conditions
Platelet aggregation
Results from 3 x 2 ANOVA showed no statistical
differences in platelet aggregation in any of the three exercise conditions at
pre-post resistance exercise neither condition x time interactions (Figure 3).
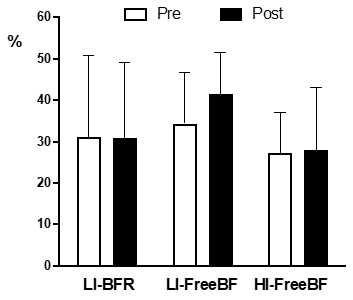
LI-BFR = low intensity with restricted blood flow; LI-FreeBF = low intensity with free blood flow; HI-FreeBF = high intensity with free blood flow; mean and
standard deviation). No differences were statistically significant for P <
0.05
Figure 3 - Platelet aggregation measured at pre- and post-exercise
under different experimental conditions
Nitrite
No significant main effects or condition x time
interactions were observed for nitrite levels. Figure 4 shows the nitrite
levels before and after the exercise bouts.
The average increment between pre
and post-exercise was 4.0 ± 1.9 4.0 ± 1.9 µM for LI-BFR, 1.1 ± 0.6 µM for an
LI-FreeBF, and 4.6 ± 5.1 µM for HI-FreeBF.
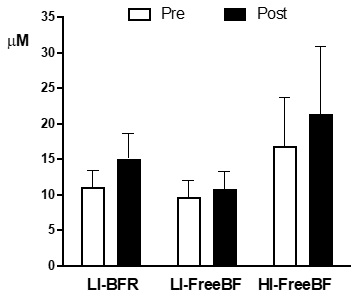
LI-BFR = low intensity with restricted blood flow; LI-FreeBF = low intensity with free blood flow; HI-FreeBF = high intensity with free blood flow; mean and
standard deviation). No differences were statistically significant for P <
0.05
Figure 4 - Blood nitrite levels measured at pre- and
post-exercise under different experimental conditions
Discussion
The present study was conducted to identify the
effects of different acute resistance exercise conditions with and without
blood flow restriction on endothelial function, blood nitrite concentrations,
and platelet aggregation. To the best of our knowledge, it is the first study
to investigate the effects of different acute BFR-RE involving major muscle
groups on endothelial function, blood nitrite concentrations, and platelet
aggregation. The main finding was that bilateral lower limbs exercises
performed under blood flow restriction did not threaten vascular function in
healthy males.
Participants of this study had homogeneous physical
and metabolic characteristics since anthropometric and lipemic profiles were
within the recommended levels for adults [15,16]. Furthermore, none of them
reported any discomfort during or after exercising on the three experimental
conditions.
Analyzing the peak shear rate (SR) values at a visual
inspection, it was possible to observe a higher increase between before and
after exercise bouts in the conditions LI-BFR (75.22 ± 203.33 s-1) and HI-FreeBF (60.63 ± 62.24 s-1), compared to LI-FreeBF values (19.78 ± 54.92 s-1). Although no
statistically significant differences were observed, numerical values of higher
increments sought in the LI-BFR and HI-FreeBF
conditions denote the high physical effort, defined by the participants'
difficulty in completing the sets and repetitions proposed under these
conditions, which may have influenced the more significant increases in the
peak SR in these conditions [17]. As well as the peak SR, the basal SR values
underwent higher numeric modifications under LI-BFR and HI-FreeBF
conditions than in LI-FreeBF. However, these changes
were not statistically significant either.
Tinken et
al. [18] demonstrated that when the exercise increases SR, an augment in
FMD is expected in response, since the higher the SR, the greater the
mechanical action of blood flow on endothelial cells, promoting the release of
vasoactive substances that promote vasodilation. Nevertheless, no significant
acute increase in FMD was observed in the present study after the three
exercise conditions. Probably the fail in identifying
significant changes was influenced by the great variability of the data.
Controversially, Paiva et al. [10] studied the
effects of FMD values 15 min after a single session of bilateral dynamic
handgrip exercise (20 min with 60% of the maximum voluntary contraction, 15
contractions per minute) and reported that the addition of BFR to the protocol
blunted the increase in FMD observed after the same exercise without BFR. The
authors attributed the findings to the higher oscillatory SR and the production
of reactive oxygen species caused by the BFR in the arm exercised under
restriction.
However, the present study did not assess shear rate
patterns or reactive oxygen species, impairing the interpretation of data.
Therefore, our findings are limited to the observation that different
intensities of resistance exercise with or without BFR produce similar
immediate effects on endothelial function.
Acute periods of augmented retrograde SR have been
observed to impair endothelial function [19]. Shear patterns in non-exercising
limbs may vary according to the different modalities of lower limb exercise
[20]. Concerning resistance exercise effects, Thomas et al. [21] reported
that three sets of 10 RM of knee extension caused only a trivial and
short-lived increase in antegrade shear rate and no significant change in
retrograde flow immediately following exercise. Less is known about RE-BFR, but
it is possible to speculate that the exercises protocols tested in the present
study should not have notably increased the retrograde SR, and therefore,
should not have had harmful effects on FMD.
Concerning platelet aggregation, no deleterious effect
was provoked by the application of BFR to resistance exercise. It is known that
part of the endothelial dysfunction is related to platelet adhesion, as
platelets are activated when there is an injury in the endothelium [22].
Although chronic physical training plays an important role in preventing
manifestations of cardiovascular diseases, including atherosclerosis [23], an
acute strenuous exercise bout increases platelets activation and aggregation,
promoting inflammatory processes [24]. An explication to the attenuation in the
inherent increase in platelet aggregation observed in the present study might
be attributed to the training status of the participants, all resistance trained.
According to Creighton et al. [25], after an acute bout of heavy
resistance exercise, platelet activation markers appear to be lower in
individuals who are resistance trained.
As stated earlier, plasma levels of nitrite, a
suitable and reliable indicator of systemic NO production [26], did not present
statistically significant changes in response to the exercise protocols
applied. These findings are according to Boeno et
al. [27], who developed a study very similar to the present one. They
compared the effect of either LI-BFR, LI-FreeBF, and
HI-FreeBF resistance exercise (upper and lower limbs)
on nitric oxide byproduct levels and antioxidant enzyme activity in healthy
young men. They demonstrated that one session of LI-BFR resistance exercise was
not capable of modulating plasma NOx levels. However, when compared with the
levels in condition HI-FreeBF, these levels were
significantly higher after exercise.
It is important to emphasize that NO has
antiatherogenic properties and exerts inhibitory actions for platelet adhesion,
activation, and aggregation [28]. From a hemostatic point of view, the
non-elevation of platelet aggregation associated with maintaining nitrite
levels may demonstrate that this method can be safe to be practiced, as it does
not increase the risk of vascular thrombus formation.
It is important to note that we used an exercise
protocol involving only lower limbs in the present study. However, earlier
studies have indicated that vascular responses to exercise may also be evident
in arteries not directly feeding the active skeletal muscle, with evident
changes in brachial artery FMD following lower limb exercises [29].
It is recognized some limitations in the
interpretation of the results of the present study. The hemodynamic responses
were examined only immediately after the exercise bout, and observation in the
time course of responses would be more appropriate. We did not assess shear
rate pattern and reactive oxygen species production, which are essential
factors influencing vascular responses. Further studies are needed to elucidate
the role of these variables in endothelial function and platelet aggregation.
Conclusion
In summary, our results indicate that lower limbs
resistance exercise in low or high intensities and with or without BFR as used
in the present study could not significantly affect the responses induced in
endothelial function, nitrite levels, and platelet aggregation. These findings
indicate that such exercise conditions do not seem to represent cardiovascular
risk from a hemostatic point of view, at least to healthy adult men.
Conflict of interest
All authors declare that there is no conflict of
interest regarding this study and manuscript.
Funding source
Gomes PS - Productivity in Research Scholarship (PQ2)
from the National Council for Scientific and Technological Development from
Brazil - CNPq; PROCIÊNCIA Scholarship sponsored by
the State University of Rio de Janeiro
Author’s contributions
Conception of the study: Gomes PSC, Meirelles CM,
Fernandes Junior ML; Study design: Meirelles CM, Gomes PSC, Fernandes Junior
ML; Data collection: Fernandes Junior ML; Biochemical analysis: Matsuura C;
Writing of the document: Meirelles CM, Gomes PSC, Fernandes Junior ML, Matsuura
C; Writing the English version of the manuscript: Gomes PSC; Final review of
the manuscript: Meirelles CM, Gomes PSC
References
- Corban MT, Lerman LO, Lerman
A. Endothelial dysfunction cardiovascular disease pathophysiology hidden in
plain sight. Arterioscler Thromb
Vasc Biol 2019;39:1272-4. doi: 10.1161/ATVBAHA.119.312836 [Crossref]
- Zhang Y, Zhang Y, Zhang H, Ye W, Korivi M.
Low-to-moderate-intensity resistance exercise is more effective than
high-intensity at improving endothelial function in adults: a systematic review
and meta-analysis. Int J Environ Res Public Health 2021;18:6723. doi: 10.3390/ijerph18136723 [Crossref]
- Haynes A, Linden MD, Robey E, Watts GF, Barrett PH, Naylor LH, Green DJ. Acute impact of different exercise modalities on arterial and platelet function. Med Sci Sports Exerc 2018;50(4):785-91. doi: 10.1249/MSS.0000000000001505 [Crossref]
- Scott BR, Loenneke JP, Slattery KM, Dascombe BJ. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med 2015;45:313-25. doi: 10.1007/s40279-014-0288-1 [Crossref]
- Green DJ. Dawson EA, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated? A meta-analysis. Hypertension 2014;63:376-82. doi: 10.1161/HYPERTENSIONAHA.113.02044 [Crossref]
- Early KS, Rockhill M, Bryan A, Tyo B, Buuck D, McGinty J. Effect of blood flow restriction training on muscular performance, pain and vascular function. Int J Sports Phys Ther 2020;15(6):892-900. doi: 10.26603/ijspt20200892 [Crossref]
- Credeur DP, Hollis BC, Welsch MA. Effects of handgrip training with venous restriction on brachial artery vasodilation. Med Sci Sports Exerc 2010;42(7):1296-302. doi: 10.1249/MSS.0b013e3181ca7b06 [Crossref]
- Ramis TR, De Muller LCH, Boeno FP, Teixeira BC, Rech A, Pompermayer MG, et al. Effects of
traditional and vascular restricted strength training program with equalized
volume on isometric and dynamic strength, muscle thickness, electromyographic
activity, and endothelial function adaptations in young adults. J Strength Cond
Res 2020;34(3):689-98 doi: 10.1519/JSC.0000000000002717 [Crossref]
- Hunt JEA, Walton LA, Ferguson RA. 2012. Brachial artery modifications to blood flow-restricted handgrip training and detraining. J Applied Physiol 112(6):956-61. doi: 10.1152/japplphysiol.00905.2011 [Crossref]
- Paiva FM, Vianna LC, Fernandes IA, Nobrega AC, Lima RM. 2016. Effects of disturbed blood flow during exercise on endothelial function: a time course analysis. Braz J Med Biol Res 2016;49(4):49. doi: 10.1590/1414-431X20155100 [Crossref]
- Ferguson RA, Hunt JE, Lewis MP, Martin NR, Player DJ, Stangier C, et al. The acute angiogenic signaling response to low load resistance exercise with blood flow restriction. Eur J Sport Sci 2018;18(3):397-406. doi: 10.1080/17461391.2017.1422281 [Crossref]
- Nascimento DC, Schoenfeld BJ, Prestes J. Potential implications of blood flow restriction exercise on vascular health: a brief review. Sports Med 2020;50(1):73-81. doi: 10.1007/s40279-019-01196-5 [Crossref]
- Ahmadizad S, El-Sayed MS,
MacLaren DP. Responses of platelet activation and
function to a single bout of resistance exercise and recovery. Clin Hemorheol Microcirc
2006;35(1-2):159-68. Available from: https://pubmed.ncbi.nlm.nih.gov/16899922/
- Shephard RJ. PAR-Q, Canadian Home Fitness Test and exercise screening
alternatives. Sports Med
1988;5(3):185-95. doi: 10.2165/00007256-198805030-00005 [Crossref]
- Haun DR, Pitanga FJ, Lessa I. Razão cintura/estatura comparado a outros indicadores antropométricos de obesidade como preditor de risco coronariano elevado. Rev Assoc Med Bras 2009;55(6):705-11. doi: 10.1590/s0104-42302009000600015 [Crossref]
- Xavier HT, Izar MC, Neto JR, Assaad MH, Rocha VZ, Sposito AC, et al. Diretriz brasileira de dislipidemias e prevenção da aterosclerose. Arq Bras Cardiol 2013;101(4):1. doi: 10.5935/abc.20170121 [Crossref]
- Morishima T, Tsuchiya Y, Iemitsu M, Ochi E. High-intensity resistance exercise with
low repetitions maintains endothelial function. Am J Physiol
Heart Circ Physiol 2018;315:H681-6. doi: 10.1152/ajpheart.00281.2018 [Crossref]
- Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, et al. Impact of shear rate modulation on vascular function in humans. Hypertension 2009;54(2):278-85. doi: 10.1161/HYPERTENSIONAHA.109.134361 [Crossref]
- Thijssen DHJ, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 2009;53(6):986-92(a). doi: 10.1161/HYPERTENSIONAHA.109.131508 [Crossref]
- Thijssen DHJ, Dawson EA, Black MA, Hopman MTE, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exer 2009;41(5):1072-9(b). doi: 10.1249/MSS.0b013e3181923957 [Crossref]
- Thomas KN, Kissling LS, Gibbons TD, Akerman AP, van Rij AM, Cotter JD. The acute effect of resistance exercise on limb blood flow. Exp Physiol 2020;105(12):2099-109. doi: 10.1113/EP088743 [Crossref]
- Stassen JM, Arnout J, Deckmyn H. The hemostatic system. Curr Med Chem 2004;11(17): 2245-60. doi: 10.2174/0929867043364603 [Crossref]
- Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol 2008;105(2):766-78. doi: 10.1152/japplphysiol.01028.2007 [Crossref]
- Ahmadizad S, El-Sayed MS. The effects of graded resistance exercise on platelet aggregation and activation. Med Sci Sports Exerc 2003;35(6):1026-32. doi: 10.1249/01.MSS.0000069406.54766.C6 [Crossref]
- Creighton BC, Kupchak BP, Aristizabal JC, Flanagan SD, Dunn-Lewis C, Volk BM, et al. Influence of training on markers of platelet activation in response to a bout of heavy resistance exercise. Eur J Appl Physiol 2013;113:2203-9. doi: 10.1007/s00421-013-2645-4 [Crossref]
- Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res 2005;39(8):797-815. doi: 10.1080/10715760500053651 [Crossref]
- Boeno FP, Ramis TR, Farinha JB, LemosLS, Medeiros NS, Ribeiro JL. Acute effects of strength exercise with blood flow restriction on vascular function of young healthy males. J Vasc Bras 2018;17(2):122-7. doi: 10.1590/1677-5449.011017 [Crossref]
- Vanni DS, Horstmann B, Benjo AM, Daher JP, Kanaan S, Sleiman M. Óxido nítrico: inibição das plaquetas e participação na formação do trombo. J Bras Patol Med Lab 2007;43(3):181-9. doi: 10.1590/S1676-24442007000300007 [Crossref]
- Zhang Y, Zhang Y-J, Zhang H, Ye WB, Korivi M. Low-to-moderate-intensity resistance exercise is more effective than high-intensity at improving endothelial function in adults: a systematic review and meta-analysis. Int J Environ Res Public Health 2021;18:6723. doi: 10.3390/ijerph18136723 [Crossref]
