Rev Bras Fisiol Exerc 2022;21(1):77-89
doi: 10.33233/rbfex.v21i1.5096REVIEW
Acute inflammatory responses to high-intensity versus moderate-intensity
exercise in young men: a systematic review
Respostas
inflamatórias agudas ao exercício de alta intensidade versus exercício de
intensidade moderada em homens jovens: uma revisão sistemática
Carlos José Nogueira1,2,
Josiana Kely Rodrigues
Moreira da Silva1,3,6, Viviane Monteiro Dias1,4, Paula
Paraguassu Brandão1,4,6, Estélio Henrique
Martin Dantas1,5
1Universidade Federal do Estado do Rio de
Janeiro (UNIRIO), Rio de Janeiro, RJ, Brazil
2Força Aérea Brasileira (FAB) / Escola
Preparatória de Cadetes do Ar (EPCAR), Barbacena, MG, Brazil
3Universidade do Estado do Pará, Belém
PA, Brazil
4Universidade Celso Lisboa (UCL), Rio de
Janeiro, RJ, Brazil
5Universidade Tiradentes (UNIT), Aracajú, SE, Brazil
6Universidade Estácio de Sá, Brazil
Received: February 16,
2022; Accepted: February
26, 2022.
Correspondence: Carlos José Nogueira, Universidade
Federal do Estado do Rio de Janeiro – UNIRIO, Laboratório de Biociências da Motricidade
Humana (LABIMH), Rua Dr Xavier Sigaud,
290/301 Praia Vermelha, Rio de Janeiro RJ.
Carlos
José Nogueira: carlosjn29@yahoo.com.br
Josiana Kely
Rodrigues Moreira da Silva: josikely@hotmail.com
Viviane
Monteiro Dias: vivimonteirodias@gmail.com
Paula
Paraguassu Brandão: pb.paula@yahoo.com.br
Estélio Henrique Martin Dantas:
estelio.dantas@unirio.br
Abstract
Background: Physical exercise has considerable effects on
inflammation markers. Objective: The aim of this review was to compare
the acute effects of high-intensity exercise and moderate-intensity exercise on
inflammation in young men. Methods: A search was conducted in the
Medline/Pubmed, Embase, Cochrane Library, Lilacs/BVS
and Web of Science databases and completed in July 2021. Studies were eligible
if they met the predefined inclusion criteria: a) randomized clinical trials
and quasi-experimental studies; b) conducted on active young men (15 to 24
years old); c) written in Portuguese, English or Spanish; d) applying intense
and/or moderate physical exercise protocols. The search strategy was built with
the following descriptors: “young adult”, “exercise”, “high-intensity interval
training”, and “inflammation”. The specific components examined included
circulating levels of cytokines IL-6, IL-10, IL-1β, and TNF-α. The
risk-of-bias in the results of the studies was assessed with the tools Rob 2
and ROBINS-I. Results: From the 1417 records identified, 5 studies were
selected for analysis (n = 96). Most studies showed a high risk-of-bias. Conclusion:
The results suggested an increase in the acute inflammatory response,
regardless of exercise intensity. It is assumed that the inflammatory response
may also have been influenced by the duration and type of exercise. Further
research is needed to examine the impact of exercise intensity on inflammation.
Keywords: exercise; inflammation; cytokines; high intensity
interval training; tumor necrosis factor alpha.
Resumo
Introdução: O exercício físico tem efeitos
consideráveis nos marcadores de inflamação. Objetivo: O objetivo desta
revisão foi comparar os efeitos agudos do exercício de alta intensidade e do
exercício de intensidade moderada na inflamação em homens jovens. Métodos:
Uma busca foi realizada nas bases de dados Medline/PubMed,
Embase, Cochrane Library, Lilacs/BVS e Web of Science e concluída em julho de 2021. Os estudos eram
elegíveis se atendessem aos critérios de inclusão predefinidos: a) ensaios
clínicos randomizados e quase-experimentais; b) realizado em homens jovens
ativos (15 a 24 anos); c) escritos em português, inglês ou espanhol; d)
aplicação de protocolos de exercícios físicos intensos e/ou moderados. A
estratégia de busca foi construída com os seguintes descritores: “adulto
jovem”, “exercício”, “treinamento intervalado de alta intensidade” e
“inflamação”. Os componentes específicos examinados incluíram níveis
circulantes de citocinas IL-6, IL-10, IL-1β e TNF-α. O risco de viés
nos resultados dos estudos foi avaliado com as ferramentas Rob 2 e ROBINS-I. Resultados:
Dos 1.417 registros identificados, 5 estudos foram selecionados para análise (n
= 96). A maioria dos estudos mostrou um alto risco de viés. Conclusão:
Os resultados sugeriram um aumento da resposta inflamatória aguda, independente
da intensidade do exercício. Supõe-se que a resposta inflamatória também pode
ter sido influenciada pela duração e tipo de exercício. Mais pesquisas são
necessárias para examinar o impacto da intensidade do exercício na inflamação.
Palavras-chave: exercício; inflamação; citocinas;
treinamento intervalado de alta intensidade; fator de necrose tumoral alfa.
Introduction
Inflammation is described as a defense response of the
body against an aggressor agent to promote healing/repair [1]. The extent of
this process is regulated by pro- and anti-inflammatory factors [2].
Physical exercise leads to a robust inflammatory
response determined by the recruitment of leukocytes and increase in the level
of circulating inflammatory markers produced by immune cells and directly from
active muscle tissue [3].
Exercise-induced changes in inflammation can be
divided into acute effects (changes during and immediately after exercising)
and chronic effects (changes in resting or baseline levels when the acute
effects induced by exercise are washed away) [3,4].
Overload during exercise causes microtraumas of
different levels in striated skeletal muscle tissue, connective tissue, and
bone tissue resulting in an acute inflammatory response, orchestrated, among
others, by neutrophils and macrophages whose function is to clean, repair and
restore previously damaged tissues [5]. The pro-inflammatory response promotes
the release of interleukin-1 beta (IL-1β) and tumor necrosis factor alpha
(TNF-α), which are expressed in the skeletal muscle, followed by the
expression of the anti-inflammatory cytokines
interleukin-6 (IL-6), interleukin 1 receptor antagonist protein (IL-1ra),
soluble TNF-α receptors and interleukin-10 (IL -10) [6].
Previous studies found different results when
comparing the acute and chronic effects of high-intensity intermittent training
(HIIT) and moderate-intensity training on the metabolic profile and
inflammatory response in adult men [2,7].
Cabral-Santos et al. [2] concluded that when
the volume of both exercise protocols equals, both promote similar inflammatory
responses, leading to an anti-inflammatory state. In contrast, the findings of
Lira et al. [7] showed that HIIT had a greater impact on the acute
response of IL-6 regardless of the training period, and an acute increase in
the post-exercise TNF-α levels, regardless of the intensity and the
training period. IL-10 increased immediately after acute exercise, regardless
of the training period and intensity.
Evidence from a recent systematic review [3] points to
an acute inflammatory response after training. TNF-α and IL-10 increased
only after intense exercise, and a greater increase in the levels of IL-6 and
IL-1β after intense exercise compared to moderate exercise. However, it is
noteworthy that the participants of the studies analyzed in the review were
moderately or highly trained adults and athletes.
Because of the scarcity of studies comparing these
training protocols in a younger population, the present study aimed to analyze
and summarize the available scientific evidence on the acute effects of
high-intensity versus moderate-intensity physical exercise on inflammatory
markers in young men.
Methods
This review was prepared in accordance with the
Cochrane Handbook for Systematic Reviews of Interventions [8] and the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses PRISMA Statement 2020
[9]. It was based on the methodological instructions for the preparation of a
systematic review and meta-analysis proposed by Martimbianco
[10]. The review protocol was registered with PROSPERO (International
Prospective Register of Systematic Reviews), nº CRD42021259733.
Search strategy
A sensitive search was performed in the databases:
Medical Literature Analysis and Retrieval System Online (Medline, via Pubmed), Embase, Cochrane Library, Latin American and
Caribbean Health Sciences Literature (Lilacs) via VHL Regional Portal and Web
of Science. We conducted a handsearch in reference
lists of included studies, after analysis of the full text, to retrieve
potentially relevant studies that had not appeared in the initial search.
Searches were conducted in July 2021.
We used Boolean operators in the research protocol,
requiring the title, abstract, or keywords to include the following Descriptors
in Health Sciences (DeCS) and Medical Subject
Headings (MeSH): “young adult”, “exercise”,
“high-intensity interval training”, and “inflammation”. Similar terms or
synonyms were used to ensure a more inclusive initial search and avoid an
overly narrow scope of the studies analyzed. The search strategies used are
presented along with the search strategy used in Medline via Pubmed adapted to other databases (Chart I).
Chart I - Strategies used for electronic searches
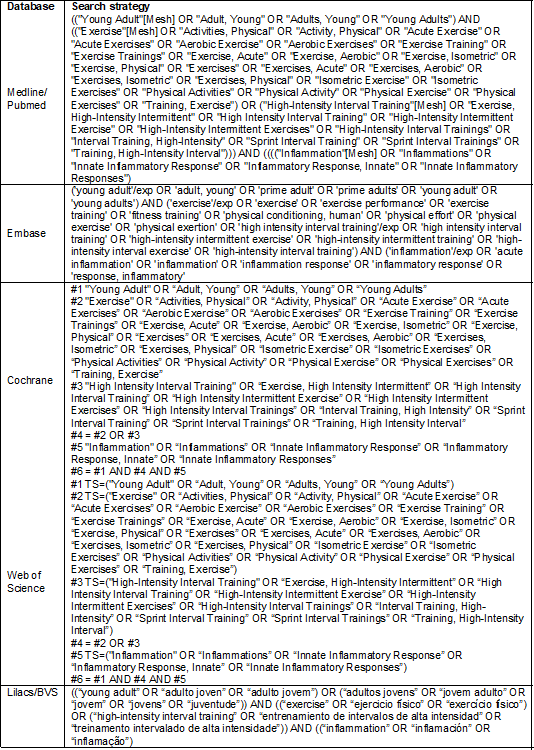
Source: Author 2021
Research question
The research question and the search strategy were
constructed using the Population, Intervention, Comparison, and Outcome (PICO)
model, common in the Evidence-Based Practice and recommended for the
development of systematic reviews [11].
From the foregoing, young men (15 to 24 years old)
[12] doing regular physical activity (at least 1 year) were selected as
“Population”; studies with intense physical exercise were considered for
“Intervention”; studies involving moderate-intensity physical exercise were
considered for “Control”; and the primary and/or secondary outcomes that
assessed acute inflammatory responses were considered as “Outcome”. Thus, the
following PICO question was constructed: Does high-intensity physical exercise
induce greater acute inflammatory responses than does moderate-intensity
exercise in young men?
Eligibility criteria
Randomized clinical trials and quasi-experimental
(non-randomized) studies published in English, Spanish and Portuguese were
included. There was no restriction on publication date or duration of studies.
The following exclusion criteria were stipulated: studies other than randomized
and nonrandomized clinical trials; studies carried out with adults (≥ 25
years), older adults, children, disabled young people, people with chronic
diseases or other limitations; studies with high performance athletes and
studies with animal models.
Study selection
Two review authors independently screened the studies,
and disagreements were resolved by consensus or deliberation with a third
reviewer. The selection of studies was carried out in two stages. At the first
stage, titles and abstracts of records retrieved in the initial search were
examined, and potentially eligible studies were pre-selected. At the second stage,
the full text of the pre-selected studies was evaluated to confirm eligibility.
The screening process was carried out using the Rayyan web application
(https://rayyan.qcri.org) [13]. The entire study screening process followed the
steps proposed by the PRISMA 2020 [9] Flowchart, as illustrated in Figure 1.
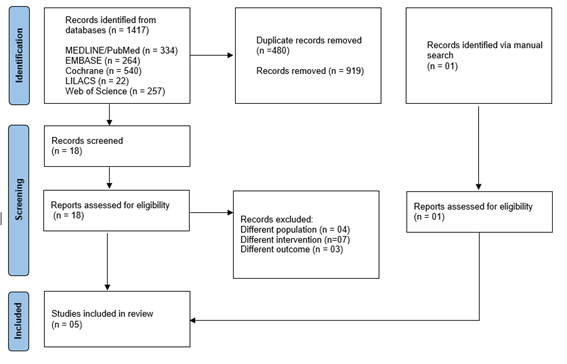
Figure 1 - Flow diagram of article selection. Adapted from:
Page et al. [9]
Data extraction
At this step, we used standardized data extraction
forms. Two reviewers independently conducted the extraction of data regarding
the methodological characteristics of the studies, interventions and outcomes.
Disagreements were resolved by consensus. Data on exercise type, exercise
intensity and duration, and exercise-induced changes in inflammation markers
were identified and evaluated. The effects of exercise intensities on
inflammatory markers were examined in blood samples collected pre- and
post-exercise and up to 72h post-exercise.
Risk-of-bias assessment
The risk of bias was assessed independently by two
reviewers using suitable tools for each study design. The Cochrane [14]
risk-of-bias tool for randomized trials (RoB 2) has
five domains, as follows: 1) bias arising from the randomization process; 2)
bias due to deviations from intended interventions; 3) bias due to missing
outcome data; 4) bias in measurement of the outcome; and 5) bias in selection
of the reported result. For non-randomized or quasi-randomized studies, the
ROBINS-I [15] tool has seven domains for assessment of bias: 1) bias due to
confounding; 2) bias in the selection of participants into the study; 3) bias
in the classification of interventions; 4) bias due to deviations from the
intended interventions; 5) bias due to missing data; 6) bias in measurement of
outcomes; and 7) bias in the selection of the reported result. The risk-of-bias
assessment of randomized clinical trials is summarized in Figure 2, and the
risk of bias of non-randomized or quasi-experimental studies is summarized in
Figure 3.
Results
The initial search identified 1417 records in the
databases. After excluding 480 duplicates, 937 studies were examined (reading
of title and abstract) and 18 studies were pre-selected for full text reading
and eligibility assessment. Fourteen studies were excluded for presenting
different populations, interventions and outcomes. One study was identified and
included through handsearching in the reference lists of the eligible studies.
Finally, 05 studies were selected for the qualitative synthesis (n = 96)
(Figure 1).
Study characteristics
The studies were categorized according to design,
exercise protocol and inflammatory markers measured. Two studies used a
randomized clinical trial design with experimental group(s) and a control group
[16,17]. The others used a non-randomized or quasi-experimental design [18,19,20],
while two studies [18,20] presented a crossover model.
The studies applied the following exercise protocols:
upper limb resistance training [16,17]; walking [18] and running on a treadmill
[20]; interval and continuous exercises on the cycle ergometer [19].
The inflammatory marker interleukin-6 was evaluated in
all studies [16,17,18,19,20], TNF-α in three studies [16,17,18] and IL-10 [20] and
IL-1β [17] in one study.
Most studies collected blood samples prior to exercise,
immediately post exercise, and at different times up to 24 hours post exercise.
However, some markers were evaluated up to 48 hours (IL-6 and TNF-α) [18]
and 72 hours after exercise (IL-6, TNFαand IL-1β) [17].
Because the studies presented distinct characteristics
of interventions such as different protocols and measurement times of
inflammatory markers, it was not possible to carry out a quantitative synthesis
among the set of studies. Therefore, a qualitative approach was more
appropriate. Chart II shows the characteristics of the study.
Chart II - Characteristics of the studies
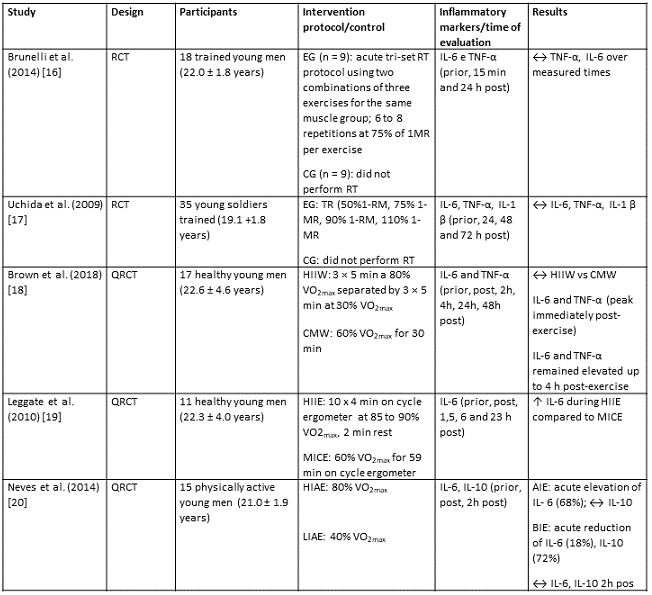
↑ = significant increase
(p < 0.05); ↓ = significant
reduction (p < 0.05); ↔ = no changes; RCT = Randomized
Clinical Trial; QRCT = Quasi-Randomized Clinical Trial; EG = Experimental
Group; CG = Control group; HIIW = High Intensity Intermittent Walking; CMW =
Continuous walking of moderate intensity; HIIE = High-intensity intermittent
exercise, MICE = Moderate-intensity continuous exercise; HIAE = High Intensity
Aerobic Exercise; LIAE = Low/moderate intensity aerobic exercise; RT =
Resistance training; MR = Maximum repetition; IL-6 = Interleukin 6; IL-1β
= Interleukin 1 beta; TNF-α = Tumor necrosis factor alpha
Risk of bias of individual studies
The Cochrane risk-of-bias tool for randomized trials RoB 2 [16,17] presented a high risk of overall bias (100%),
taking into account the worst assessment for each study (Figure 2). Both
studies had problems with randomization, that is, the authors did not report
how the randomization was performed and whether the allocation sequence was
adequately concealed. The studies were considered as having high risk of bias
[16] and a number of concerns [17] due to deviations from the intended
intervention, as participants and instructors were aware of the interventions
provided to the groups of the research.
Uchida et al. [17] investigated plasma
concentrations of IL-1β IL-6 and TNF-α and found them not detectable
for some participants even after exercise, resulting in a high risk of bias due
to missing outcome data. There was a low risk of bias resulting from the
measurement of outcomes and selection of reported results in the two randomized
clinical trials [16,17]. This estimation derives from the use of appropriate methods
of outcome measurement, with no difference between the experimental and control
groups. There was no evidence of publication bias in the two studies analyzed.
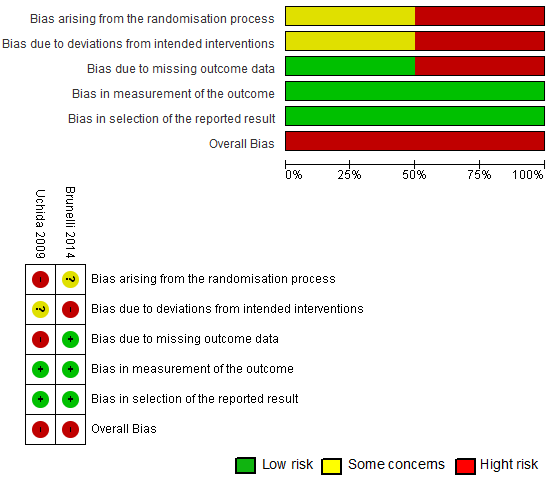
Figure 2 - Percentage distribution and risk-of-bias
scenario in individual studies (Rob2) [14]
In the assessment carried out with the ROBINS-I
(Figure 3), two quasi-experimental studies [18,19] had severe risk of overall
bias considering the worst evaluation for each study. Both studies had no
control group (rest or did not perform the compared training), which probably
implied a high risk of bias on D3. However, the study by Neves et al.
[20] was found as having a moderate risk of bias, considering that the it
presented a low or moderate risk of bias for all domains.
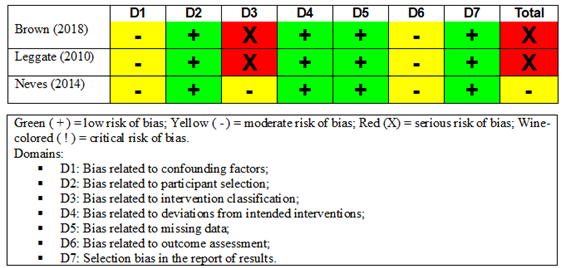
Figure 3 - Summary of risk of bias in non-randomized
studies using the ROBINS-I [15] tool
Discussion
The analysis of individual studies with resistance
training of different intensities for upper limbs showed that there were no
significant changes in plasma concentrations of proinflammatory cytokines after
exercise over the measurement times, regardless of intensity. IL-6 and TNF-α
showed trivial to small effect sizes after high-intensity resistance training
(tri-set) (75% of 1MR) compared to control [16]. There were also no significant
changes in IL-6, TNF-α and IL-1β markers after bench press training
at different intensities and the same volume (50% of 1MR, 75% of 1MR, 90% of
1MR and 110% of 1MR) [17].
In contrast to the findings of this analysis, the
concentration of IL-6 significantly increased after high-intensity resistance
training in healthy adult men [21,22] and trained and untrained men [23], with
increased levels up to one hour post-exercise [21,23].
Another study showed a significant increase in the
plasma concentration of TNF-α up to 2 hours after both high volume and
high intensity and low volume and high intensity resistance exercises [24]. Ihalainen et al. [25] observed that IL-1β
increased immediately after high-intensity resistance exercise (80% of 1MR) in
adults. It is noteworthy that these studies evaluated different muscle groups
in adult individuals with different training statuses, which may partially
explain the different results.
Apparently, resistance exercise-induced inflammation
does not result in large increases in pro-inflammatory cytokines such as
high-intensity aerobic and intermittent exercises [7,18,19,20,26]. Factors other
than muscle damage, including exercise duration, energy requirements, and
oxidative stress are assumed to determine the size of the cytokine response
[3,17].
Studies using different protocols of aerobic and
intermittent exercises showed conflicting results [18,19,20]. Brown et al.
[18] found significant increases in the pro-inflammatory cytokines IL-6 and
TNF-α after high- and moderate-intensity walking exercises. The peak of
these cytokines occurred immediately after exercise and remained elevated until
at least 4 hours post-exercise. Thus, the exercise of walking, regardless of
the intensity, promoted a systemic increase in the concentrations of IL-6 and TNF-α.
These results are consistent with the findings of
Cyprian et al. [27] for the serum concentration of IL-6, which did not
change significantly when comparing pre- and post-exercise values in both
intervention groups (continuous vs. intermittent), as well as in the control
group within at least 5 hours after rest. In another study, TNF-α
increased immediately after the acute exercise session, despite the intensity
of different protocols of intermittent exercise in adults [7].
However, Leggate et al.
[19] showed that high-intensity intermittent exercise (HIIT) caused a
significantly greater increase in IL-6 concentrations than continuous
moderate-intensity exercise. Similar results were found in other studies
[7,26].
Lira et al. [7] observed a significant increase
in IL-6 immediately after a session of HIIT compared with the acute and chronic
effects of HIIT versus continuous training of moderate intensity in physically
active adults. The plasma IL-6 response to exercise was found significantly
higher after high-intensity interval exercise than low-intensity exercise in
adult men and women [26].
Nevertheless, Leggate et
al. [19] obtained contrary results from the study by Cabral-Santos et
al. [2], who demonstrated that both exercise protocols (HIIT versus
moderate continuous), for a corresponding volume, promote similar inflammatory
responses, leading to an anti-inflammatory state.
Neves et al. [20] compared aerobic exercises of
different intensities and observed a greater acute response of IL-6 after the
high-intensity protocol than the aerobic exercise of low to moderate intensity.
The concentration of IL-10, on the other hand, showed a greater acute systemic
reduction for the aerobic exercise of low to moderate intensity compared to
aerobic exercise of high intensity immediately after strain [20].
These results for IL-6 are different from the findings
of Pozzolo et al. [28], in which IL-6 showed
no variation between pre- and post-exercise in the two aerobic exercise sessions
with different intensities, nor in the comparison between one session and
another. However, they are consistent with IL-10 concentrations, which
significantly reduced in continuous low-intensity aerobic exercise [28]. It is
assumed that less intense exercise protocols are associated with a lower
anti-inflammatory response and that there is no change in the anti-inflammatory
activity when the exercise intensity is increased [28].
The evidence, however, needs to be interpreted
considering some limitations. The first is due to the small size of the total
sample (n = 96) of participants in the reviewed studies, which may have
affected the interpretation and reproducibility of the results. Among other
factors, differences in design, experimental and control protocols, outcome
measures, and the missing outcome data in one study prevented further
quantitative synthesis. The conclusions were based on data of relatively low
quality and therefore high risk of bias. Important methodological issues such
as lack of information on randomization and allocation sequence concealment
limited the strength of the conclusions of the studies included. Finally, the
results of this review cannot be extrapolated to the general population, as it
analyzed only young individuals.
Conclusion
Overall, the analysis of individual studies showed an
acute inflammatory response post exercise, with increase in most
pro-inflammatory markers. However, the increases are independent of exercise
intensity in a younger population, especially when resistance exercise
protocols are used. Furthermore, we believe that the acute inflammatory
response may also have been influenced by the duration and type of exercise.
Therefore, due to limitations and inconsistency in the evidence found, the
results must be interpreted with caution.
Future research of greater methodological quality,
capable of associating intensity with volume and type of training, as well as
separately clustering other age groups, may clarify the results found so far.
Conflict of interest
No conflicts of interest have been reported for this
article.
Financing source
There were no external sources of funding for this
study.
Authors’ contributions
Conception and design of the research: Nogueira CJ; Data collection: Nogueira CJ, Brandão
PP, Silva JKRM, Dias VM; Data analysis and interpretation: Nogueira CJ, Brandão PP, Silva JKRM, Dias VM; Statistical analysis:
Not applicable; Obtaining Financing: Not applicable; Manuscript
writing: Nogueira CJ, Dantas EHM; Critical
review of the manuscript: Nogueira CJ, Brandão
PP, Silva JKRM, Dias VM; Final review of the manuscript: Nogueira CJ, Brandão PP, Dantas EHM.
Academic link
This study is linked to the thesis of doctoral student
Nogueira CJ, from the Stricto Sensu
Post-Graduation Program in Nursing and Bioscience, Universidade
Federal do Estado do Rio de Janeiro (UNIRIO), Rio de Janeiro, RJ, Brazil
References
- Scheffer D, Latini A. Exercise-induced immune system response:
Anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta
Mol Basis Dis
2020;1866(10):165823. doi: 10.1016/j.bbadis.2020.165823 [Crossref]
- Cabral-Santos C, Gerosa-Neto J, Inoue DS, Panissa VL, Gobbo LA, Zagatto AM,
et al. Similar anti-inflammatory acute responses from
moderate-intensity continuous and high-intensity intermittent exercise. J
Sports Sci Med [Internet] 2015 [cited 2022 Mar 14];14:849-56.
Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4657429/
- Cerqueira E, Marinho DA, Neiva HP, Lourenço O. Inflammatory effects of high and moderate intensity exercise. A systematic review. Front Physiol 2020;10:1550. doi: 10.3389/fphys.2019.01550 [Crossref]
- Pedersen BK. The physiology of optimizing health with a focus on exercise
as medicine. Annu Rev Physiol
2019;81:607-27. doi: 10.1146/annurev-physiol-020518-114339 [Crossref]
- Silva FOC, Macedo DV. Exercício físico, processo inflamatório e adaptação: uma visão geral. Rev Bras Cineantropom Desempenho Hum 2011;13(4):320-8. doi: 10.5007/1980-0037.2011v13n4p320 [Crossref]
- Apostolopoulos NC. Stretch intensity and the
inflammatory response: a paradigm
shift. Switzerland AG: Springer Nature;
2018.
- Lira FS, Santos T, Caldeira RS, Inoue DS, Panissa V, Cabral-Santos C, et al. Short-term high- and moderate-intensity training modifies inflammatory and metabolic factors in response to acute exercise. Front Physiol 2017;8:856. doi: 10.3389/fphys.2017.00856 [Crossref]
- Higgins JPT, Thomas J, Chandler J, Cumpston M,
Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions
version 6.0 (updated July 2019). Cochrane, 2019 [Internet]. [cited 2022 Mar
14]. Available from: http://www.training.cochrane.org/handbook
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed.) 2021;372(71). doi: 10.1136/bmj.n71 [Crossref]
- Martimbianco ALC. How to prepare a systematic review and metaanalysis: the methodological approach. Motriz: J Phys Ed 2021;27:e10200227. doi: 10.1590/S1980-657420210000227 [Crossref]
- Santos CMDC, Pimenta CADM, Nobre MRC. The PICO strategy for the research question construction and evidence search. Rev Latinoam Enferm 2007;15(3):508-11. doi: 10.1590/S0104-11692007000300023 [Crossref]
- WHO. The health of youth. Geneva, 1989
(document A42/Technical Discussions/2). [cited 2021 Jul 15]. Available from:
http://apps.who.int/iris/bitstream/handle/10665/173082/WHA42_TD-6_eng.pdf?sequence=1
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan: a web and mobile app for systematic reviews. Syst Rev 2016;5(1)210. doi: 10.1186/s13643-016-0384-4 [Crossref]
- Sterne JA, Savović J, Page M J, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.) 2019;366. doi: 10.1136/bmj.l4898 [Crossref]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Online) 2016;355. doi: 10.1136/bmj.i4919 [Crossref]
- Brunelli DT, Caram K, Nogueira FR, Libardi CA, Prestes J, Cavaglieri CR. Immune responses to an upper body tri-set resistance training session. Clin Physiol Funct Imaging 2014;34(1),64-71. doi: 10.1111/cpf.12066 [Crossref]
- Uchida MC, Nosaka K, Ugrinowitsch C, Yamashita A, Martins E, Jr Moriscot AS, et al. Effect of bench press exercise intensity on muscle soreness and inflammatory mediators. J Sports Sci 2009;27(5):499-507. doi: 10.1080/02640410802632144 [Crossref]
- Brown M, McClean CM, Davison GW, Brown J, Murphy MH. The acute effects of walking exercise intensity on systemic cytokines and oxidative stress. Eur J Appl Physiol 2018;118(10):2111-20. doi: 10.1007/s00421-018-3930-z [Crossref]
- Leggate M, Nowell MA, Jones SA, Nimmo MA. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress & Chaperones 2010;15(6):827-33. https//doi.org/10.1007/s12192-010-0192-z [Crossref]
- Neves PRS, Tenorio TRS, Muniz MTC, Neto LMV, Botero JP, Oyama LM, et al. Efeitos de diferentes intensidades de exercício sobre a concentração sérica de interleucinas. Rev Bras Ed Fís Esporte 2014;28(4):545-52. doi: 10.1590/1807-55092014000400545 [Crossref]
- Agostinete RR, Rossi FE, Magalhaes AJ, Rocha AP, Parmezzani SS, Gerosa-Neto J, et al. Immunometabolic responses after short and moderate rest intervals to strength exercise with and without similar total volume. Front Physiol 2016;7:444. doi: 10.3389/fphys.2016.00444 [Crossref]
- Rossi FE, Gerosa-Neto J, Zanchi NE, Cholewa JM, Lira FS. Impact of short and moderate rest intervals on the acute immunometabolic response to exhaustive strength exercise: part I. J Strength Cond Res 2016;30(6),1563-9. doi: 10.1519/JSC.0000000000001189 [Crossref]
- Ashtary-Larky D, Lamuchi-Deli N, Milajerdi A, Bakhtiar Salehi M, Alipour M, Kooti W, et al. Inflammatory and biochemical biomarkers in response to high intensity resistance training in trained and untrained men. Asian J Sports Med 2017;8(2):e13739. doi: 10.5812/asjsm.13739 [Crossref]
- Wells AJ, Hoffman JR, Jajtner AR, Varanoske AN, Church DD, Gonzalez AM, et al. Monocyte recruitment after high-intensity and high-volume resistance exercise. Med Sci Sports Exerc 2016;48(6):1169-78. doi: 10.1249/MSS.0000000000000878 [Crossref]
- Ihalainen JK, Ahtiainen JP, Walker S, Paulsen G, Selänne H, Hämäläinen M, et al. Resistance training status modifies inflammatory response to explosive and hypertrophic resistance exercise bouts. J Physiol Biochem 2017;73(4),595-604. doi: 10.1007/s13105-017-0590-0 [Crossref]
- Cullen T, Thomas AW, Webb R, Hughes MG. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: the effect of exercise intensity and volume. Appl Physiol Nutr Metab 2015;41(8):803-8. doi: 10.1139/apnm-2015-0640 [Crossref]
- Cipryan L, Svagera Z, Vala R. IL-6 and CRP
response to maximal exercise intervention. J Sports Med Phys Fitness [Internet]
2015[cited 2022 Mar 14];55(7-8):813-23. Available from:
https://www.minervamedica.it/en/journals/sports-med-physical-fitness/article.php?cod=R40Y2015N07A0813
- Pozzolo BA, Fonseca VF, Guedes AA, Oliveira G I, Dietrich D, Lima EM, et al. Efeito agudo do exercício aeróbico de diferentes intensidades em citocinas de universitários. Rev Bras Med Esporte 2020;26(6):493-7. doi: 10.1590/1517-869220202606223616 [Crossref]
