Rev Bras Fisiol Exerc 2022;21(1):5-14
doi: 10.33233/rbfex.v21i1.5120REVIEW
Acute hemodynamic modulation caused by handgrip exercise
Modulação hemodinâmica
aguda provocada pelo exercício de handgrip
Josias Melo Leite1,
Alice Miranda de Oliveira2,3,4, Marvyn de
Santana do Sacramento1,2,5, Pedro Elias Santos Souza2,3,
Luan Araújo de Pinho6, Jefferson Petto1,2,4,6
1Escola Bahiana
de Medicina e Saúde Pública (EBMSP), Salvador, BA, Brasil
2Actus Cordios
Serviço de Reabilitação Cardiovascular e Metabólica, Salvador, BA, Brasil
3Universidade Católica do Salvador
(UCSAL), Salvador, BA, Brasil
4Faculdade Centro de Treinamento
Acadêmico (CTA), São Paulo, SP, Brasil
5Faculdade Adventista da Bahia, Capoeiruçu, BA, Brasil
6Centro Universitário UniFTC,
Salvador, BA, Brasil
Received: March 4,
2022; Accepted: March 4,
2022.
Correspondence: Josias Melo Leite, Caminho 23, casa
09, conjunto Feira V, bairro Mangabeira, Feira de Santana, BA, Brazil. nino.melo@outlook.com
Josias
Melo Leite: nino.melo@outlook.com
Alice
Miranda de Oliveira: alicemofisio@gmail.com
Marvyn de Santana do
Sacramento: marvynsantana@gmail.com
Pedro
Elias Santos Souza: peedroefisio@gmail.com
Luan
Araújo de Pinho: luanpharaujo@gmail.com
Jefferson Petto: petto@cardiol.br
Abstract
Introduction: The chronic effects of Hand Grip (HG) are already
consolidated in the literature, however, the studies that evaluated the acute
effects of this intervention are heterogeneous in relation to intervention
protocols and sample characteristics (gender and age). Objective: This
study aimed to describe the acute responses of SBP, DBP, HR and Double Product
(DP) through isometric exercises with GH. Methods: This is a systematic
literature review based on the criteria of the Preferred Reporting Items
guideline for Systematic Reviews and Meta-Analyses (PRISMA), registered in
PROSPERO under id: CRD42021238275. Results: A total of 619 studies were
found in the databases and 6 in the gray literature, totaling 625 studies.
After using the eligibility criteria, 5 articles were included in this review. Conclusion:
We verified that the GH promotes an increase in HR, SBP, DBP and DP, with this
increase being more accentuated, the greater the duration and intensity of the
protocol. However, HR has a smaller increase in elderly people when compared to
young individuals.
Keywords: physical exercise; blood pressure; heart rate determination;
hand strength.
Resumo
Introdução: Os efeitos crônicos do Hand Grip (HG) já estão consolidados na literatura, entretanto,
os estudos que avaliaram os efeitos agudos dessa intervenção são heterogêneos
em relação aos protocolos de intervenção e as características amostrais (sexo e
idade). Objetivo: O presente estudo teve como objetivo descrever o
efeito agudo que o HG promove sobre a Pressão Arterial Sistólica (PAS), Pressão
Arterial Diastólica (PAD), Frequência Cardíaca (FC) e Duplo Produto (DP) em
indivíduos sadios. Métodos: Trata-se de uma revisão sistemática da
literatura baseada nos critérios do Preferred Reporting Items guideline for Systematic Reviews and Meta-Analyses (PRISMA),
com registro no PROSPERO sob id: CRD42021238275. Resultados: Foram
encontrados um total de 619 estudos nas bases de dados e 6 na literatura
cinzenta, totalizando 625 estudos. Após a utilização dos critérios de
elegibilidade, 5 artigos foram incluídos nesta revisão. Conclusão:
Verificou-se que o HG promove aumento da FC, PAS, PAD e DP, sendo essa elevação
mais acentuada, quanto maior for a duração e a intensidade do protocolo.
Entretanto, a FC apresenta menor acréscimo em idosos quando comparados a
indivíduos jovens.
Palavras-chave: exercício físico; pressão arterial; frequência
cardíaca; força da mão.
Introduction
The Hand Grip (HG) is a training alternative that
emerged around the 1970s as an intervention tool to assist in the diagnosis of
cardiovascular changes [1,2]. Clinically, isometric training with HG has been
used for the treatment of Systemic Arterial Hypertension, its effects in a
chronic form are able to reduce the levels of Systolic Blood Pressure (SBP) and
Diastolic Blood Pressure (DBP) [3], since the hemodynamic effects of this
training point to an increase in SBP, DBP and Heart Rate (HR).
Acute changes are regulated by a feedback system
involving the central nervous system. The afferent pathways of the nervous
system receive information from muscle mechanoreceptors and metaboreceptors
(type III and IV nerve fibers) promoting the reflex of pressure elevation to
exercise, through the modulation of sympathetic tone, a factor that adjusts
blood pressure (BP), FC, DP [4,5,6]. However, these variables seem to depend
directly on the volume and intensity variables used in the protocols and on the
age of the individuals [7,8,9].
Studies that assess acute effects are scarce and
heterogeneous in terms of intervention protocols and sample characteristics
(gender and age). Therefore, the aim of the present study is to describe the
acute effect that HG promotes on SBP, DBP, HR and PD in healthy individuals.
Methods
The present study is a systematic literature review
based on the criteria of the Preferred Reporting Items guideline for Systematic
Reviews and Meta-Analyses (PRISMA) [10]. The searches took place between
September and November 2021 in the following databases: Medline via Pubmed, Cochrane Library, Scientific Electronic Library
Online (Scielo), Virtual Health Library (BVS) and
Physiotherapy Evidence Database (PEDro). Google
Scholar and the references of the selected works were also checked in order to find
other studies related to the topic. This revision is registered in PROSPERO
under id: CRD42021238275.
Eligibility criterion
We considered eligible clinical trials with or without
randomization and cross-sectional studies with intervention that evaluated
adult and/or elderly individuals (18 to 80 years old) of both sexes, submitted
to different intensities of isometric training with HG and/or submitted to
dynamic physical exercise without the use of HG. The outcomes observed in the
studies involved the acute effects of training with HG on the hemodynamic
variables HR, SBP, DBP and PD. Studies composed of individuals with
cardiovascular diseases, orthopedic and/or autoimmune pathologies were not
considered eligible.
Study search and selection strategy
For the search, the Medical Subject Headings (MeSH) terms were crossed: “Hand Strength” AND “Hemodynamic”
with the respective synonyms. In the Portuguese language databases, the same
searches were repeated using the Health Sciences Descriptors (DeCS). No restrictions on publication period or language.
The searches and sorting of articles were carried out
by 2 reviewers independently, initially by titles and abstracts. Subsequently,
all articles that met the selection criteria of at least one of the reviewers
were taken for full text reading. Duplicates were identified and manually
removed by the same reviewers.
Data synthesis
After confirming the selected articles, the data were
transferred to a spreadsheet previously prepared by the authors. Disagreements
about the selection of studies and/or about the extracted data were discussed
among the researchers. Data extraction sought information about the sample, HG
intervention protocol, methods of measuring BP, HR, PD, and main outcomes in
the participants' hemodynamics.
Quality of evidence and risk of bias
The risk of bias in each study was achieved using the
Downs and Black risk of bias tool [11]. It was evaluated by 2 independent
authors and discrepancies were discussed and judged by a third author. This
checklist is a valid checklist suitable for evaluating randomized and
non-randomized studies as it provides an overall score for study quality and
the profile of scores that go beyond report quality, external and internal validity
and study power.
Results
According to the proposed methodological strategy, a
total of 619 studies were found in the databases and 6 in the gray literature,
totaling 625 studies. After using the eligibility criteria, 5 articles were
included in this review. Figure 1 presents a detail of the selection of
articles.
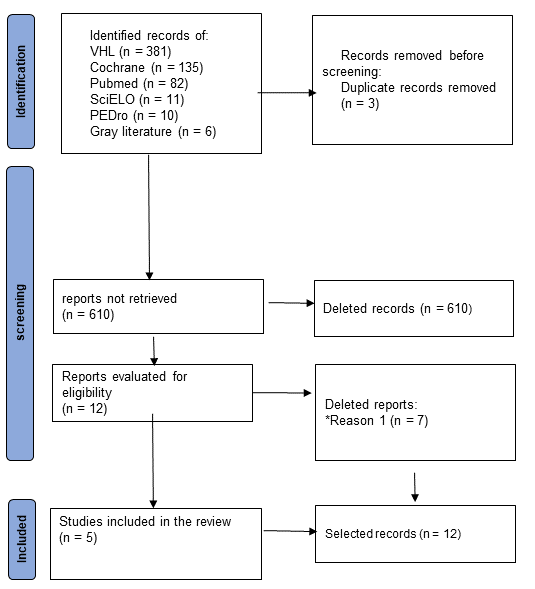
*Reason 1: Not obtaining hemodynamic variables (BP, HR
and/or PD) as the primary outcome, not using HG as an intervention, or not
assessing the hemodynamic effects of HG acutely
Figure 1 - Flowchart of article selection
The samples of the selected studies comprised 23 to 62
volunteers, totaling 198 individuals, of which 117 were male. The age of the
sample ranged from 18 ± 0.66 to 71 ± 5.6 years, the time under voltage of the
HG varied from 30 sec to 8 min, in addition to the different measurement intervals
of hemodynamic parameters, which fluctuated during the protocol around 30 sec.
at 60 sec, and immediately after intervention at 30 min. Of the 5 studies
included, only 1 was a randomized clinical trial, 3 were uncontrolled trials
and 1 was a cross-sectional study. Table I presents the methodological aspects
and the results of the 5 studies that make up the present review.
Table I - Qualitative synthesis of selected articles (PDF
annexed)
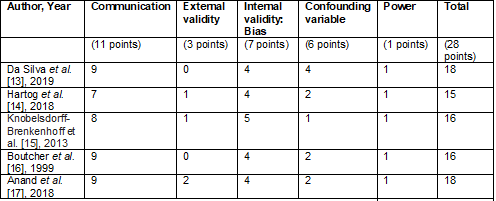
The scale proposed by Downs and Black [11] was used to
assess the quality of evidence and risk of bias in the studies included in the
qualitative synthesis. The results of its different domains can be seen in
Chart 1.
Chart 1 - Quality of evidence using the Downs and Black
scale [11]
Discussion
The present review found that the acute hemodynamic
responses (HR, SBP, DBP and PD) to HG vary according to the duration and
intensity of the protocol, age of the sample and time of evaluation. In
general, the studies pointed to an increase in SBP [13,14,15], DBP [13,14,15,16], HR [14,15,16]
and DP [14,15] during the intervention protocol and soon after its end. A
limitation to this analysis is presented by the heterogeneity of the studies
(different intervention protocols and sample characteristics, such as gender
and age), in addition to the small sample included in the present review.
In view of the above, it is interesting at this point
to discuss individually the influence of each variable of the intervention
protocol on the results obtained and later the influence of the sample characteristic
on the results obtained.
The intensity expressed by the percentage of FPmax, time under tension and the interval between sets
varied between 30%-100%, 30 seconds to 8 minutes and from 0 to 1 minute,
respectively [12,13,14,15,16], and the volume expressed by the number of series were
from one CVM to four isometric contractions. In our findings, we identified an
increase in HR, SBP, DBP, and DP during the intervention protocol [13,14,15,16] and
no changes in BP after 15 and 30min of application of the protocols at
different intensities (30%, 50% and 3% of the CVM).
Due to the influence of time under tension and the
interval between sets, the hemodynamic effects are greater in the presence of a
shorter interval and longer time under tension [7,8,9]. The mechanism that helps
us to elucidate this response is the metaboreflex,
which on the action of mechanoreceptors and muscle metaboreceptors (type III
and IV nerve fibers) mediated through modulation of sympathetic tone, controls
BP, HR, PD and peripheral vascular resistance [5,6]. These factors promote the
hemodynamic changes found in the studies [13,14,15,16], not being observed
post-intervention [12] due to a rapid modulation of the autonomic nervous
system (withdrawal of sympathetic tone and increase of parasympathetic tone)
[17]. Although these results are present in healthy individuals, in patients
with heart disease the response is possibly not the same, since in this
population the sympathetic activity is increased, which would consequently lead
to a longer recovery time of these post-exercise variables [6,8,18].
Despite the greater influence of the intervention
protocol on the outcome, two points within the studies that we evaluated
deserve to be highlighted (gender and age).
The influence of age on hemodynamic effects is shown
in the findings of Hartog et al. [13] and Boutcher
et al. [15]. Among our analyses, the older the age, the lower the HR
elevation and the greater the BP and DP elevation. Corroborating these studies Goldstraw et al. [19] when evaluating young (30
years old) and elderly (73 years old) individuals in different occasions and
tensions in the HG, found statistically significant differences in SBP (p <
0.001) and DBP (p < 0.05) during the protocol, with the highest values in
the elderly group with the exception of HR. Such results reflect that the older
the age, the more expressive are the effects on BP, with increased
vasoconstrictor responsiveness due to sympathetic stimulation and endothelial
dysfunction that affects endothelium-dependent vasodilation, the variables with
the greatest influence on this outcome [20,21]. However, the decrease in HR
over the years may occur due to a decrease in sensitivity to myocardial-related
beta-adrenergic activity [15,22].
According to Bassareo and Crisafulli [23] and Maruf et al. [24]), the responses
presented between men and women regarding hemodynamic parameters (HR and BP) do
not differ between groups when equated with body composition and physical
training status. However, the studies included in this review that evaluated
both genders did not compare the hemodynamic differences between the sexes
[13,14], however, when comparing women in different phases of the menstrual
cycle (luteal phase and follicular phase), it was observed that in the lutea,
BP and HR are higher than in the follicular phase, as demonstrated by Anand et
al. [16] when finding higher hemodynamic parameters (HR, SBP and DBP) in
women in the luteal phase compared to the follicular phase (p < 0.05), with
static isometric handgrip exercise at 30% of MVC for up to 4 min. Pivarnik et al. [25] suggest that thermoregulation
in the luteal phase is compromised, which may promote greater body heating,
when compared to the follicular phase. Thus, we assume that the change in HR
and BP starts through the mediation of thermoreceptors that transduce the
stimulus to the CNS, which by efferent pathways stimulate the effector system
(cardiovascular system and sweat glands) to balance the disorder, promoting
vasodilation and an increase in HR with consequent increase in SBP and increase
in sweating rate [26].
The hemodynamic responses addressed in the present
review are seen in sedentary or irregularly active individuals. It is believed
that active individuals present smaller responses than those found in the
results. These findings help to elucidate the hemodynamic influence of HG in
different protocols, ages and genders.
Conclusion
Hangrip
promotes an increase in HR, SBP, DBP and DP and this increase is directly
related to the duration and intensity established in the protocol. Despite the
elevation during the effort phase, no maintenance of hemodynamic changes was
observed after 15 minutes.
Academic affiliation
This article represents part of the Master's thesis by
Josias Melo Leite, supervised by Professor Jefferson
Petto Escola Bahiana de Medicina
e Saúde Pública (EBMSP),
Salvador, BA, Brazil.
Potential conflict of interest
No conflict of interests has been reported for this
article.
Funding source
The present study was carried out without funding.
Authors’ contributions
Conception and design of the research: Leite JM, Oliveira AM, Petto J; Data
collection: Leite JM, Oliveira AM; Analysis
and data interpretation: JM Leite,
Sacramento MS, Souza PES, Pinho LA; Writing
of the manuscript: Leite JM, Oliveira AM,
Souza PES; Pinho LA; Critical review of the
manuscript regarding important intellectual content: Sacramento MS, Petto
J.
References
- Richard H, Helfant MD, Maria A, Devilla MD, Steven G, Muter MD. Effect of sustained
isometric handgrip exercise on left ventricular performance. Circulation
1971;44(6):982-93. doi: 10.1161/01.cir.44.6.982 [Crossref]
- Fisher MJ, Nutter DO, Jacobs W, Schlant RC. Haemodynamic responses to isometric exercise (handgrip) in patients with heart disease. Br Heart J 1973;35(4):422-32. doi: 10.1136/hrt.35.4.422 [Crossref]
- ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA.
Guideline for the prevention, detection, evaluation, and management of high
blood pressure in adults: executive summary: A Report of the American College
of Cardiology/American Heart Association Task Force on Clinical Practice
Guidelines. Hypertension 2018;71(6):1269-1324. doi: 10.1161/HYP.0000000000000066 [Crossref]
- Mitchell JH. Neural control of the circulation during exercise: insights from the 1970-1971 Oxford studies. Exp Physiol 2012;97(1):14-9. doi: 10.1113/expphysiol.2011.058156 [Crossref]
- Nóbrega ACL, O’Leary D, Silva BM, Marongiu E, Piepoli MF, Crisafulli A. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int 2014;2014:478965. doi: 10.1155/2014/478965 [Crossref]
- Crisafulli A. The impact of cardiovascular diseases on cardiovascular regulation during exercise in humans: studies on metaboreflex activation elicited by the post-exercise muscle ischemia method. Curr Cardiol Rev 2017;13(4):293-300. doi: 10.2174/1573403X13666170804165928 [Crossref]
- Brum PC, Forjaz CLM, Tinucci T, Negrão CE. Adaptações agudas e crônicas do
exercício físico no sistema cardiovascular. Rev Paul Educ Fís [Internet] 2004 [cited 2022 March 3];18:21-31.
Available from: http://www.luzimarteixeira.com.br/wp-content/uploads/2009/09/arquivo-adaptacoes-musculares-ao-exercicio-fisico.pdf
- Mitchell JH, Wildentha K. Static (isometric) exercise and the heart: physiological and clinical considerations. Annu Rev Med 1974;25:369-81. doi: 10.1146/annurev.me.25.020174.002101 [Crossref]
- Seals RD. Influence of muscle mass on sympathetic neural activation during isometric exercise. J Appl Physiol (1985) 1989;67(5):1801-6. doi: 10.1152/jappl.1989.67.5.1801 [Crossref]
- Galvão TF, Pansani TSA, Harrad D. Principais
itens para relatar revisões sistemáticas e meta-análises: A recomendação
PRISMA. Epidemiol Serv Saúde
2015;24(2):335-42. doi: 10.5123/S1679-49742015000200017 [Crossref]
- Downs SH, Black N. The feasibility of creating a checklist for the
assessment of the methodological quality both of randomised
and non-randomised studies of health care
interventions. J Epidemiol Community Health 1998;52(6):377-84. doi: 10.1136/jech.52.6.377 [Crossref]
- Silva IM, Leonardo Sobrinho MF, Ritti-Dias RM, Sobral BPSV, Pirauá ALT, Oliveira LMFT, et al. Cardiovascular responses after isometric handgrip exercise at different intensities in healthy men. J Phys Educ 2019;30,e3020. doi: 10.4025/jphyseduc.v30i1.3020 [Crossref]
- Hartog R, Bolignano D, Sijbrands E, Pucci G, Mattace-Raso F. Short-term vascular hemodynamic responses to isometric exercise in young adults and in the elderly. Clinical Interventions in Aging 2018:13 509-14. doi: 10.2147/CIA.S151984 [Crossref]
- von Knobelsdorff-Brenkenhoff F, Dieringer MA, Fuchs K, Hezel F, Niendorf T, Schulz-Menger J. Isometric handgrip exercise during cardiovascular magnetic resonance imaging: set-up and cardiovascular effects. J Magn Reson Imaging 2013;37(6):1342-50. doi: 10.1002/jmri.23924 [Crossref]
- Boutcher SH, Stocker D. Cardiovascular responses to light isometric and aerobic exercise in 21- and 59-year-old males. Eur J Appl Physiol 1999;80:220-6. doi: 10.1007/s004210050585 [Crossref]
- Anand NS, Goudar SS. Cardiovascular responses to sustained isometric hand grip during different phases of menstrual cycle- A cross-sectional study. Indian Journal of Clinical Anatomy and Physiology, 2018;5(3):361-5. doi: 10.18231/2394-2126.2018.0084 [Crossref]
- Petrofsky JS, Lind A. Isometric strength, endurance, and the blood pressure and heart rate responses during isometric exercise in healthy men and women, with special reference to age and body fat content. Pflugers Arch 1975;360(1):49-61. doi: 10.1007/BF00584326 [Crossref]
- Samuel TJ, Beudry R, Haykowsky MJ, Sarmas S, Nelson MD. Diastolic stress testing: similarities and differences between isometric handgrip and cycle echocardiography. J Appl Physiol 2018;125:529-35. doi: /10.1152/japplphysiol.00304.2018 [Crossref]
- Goldstraw PW, Warren DJ. The effect of age on the cardiovascular responses to isometric exercise: A test of autonomic function. Gerontology 1985;31:54-8. doi: 10.1159/000212681 [Crossref]
- Taddei S, Virdis A, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 2000;101:2896-2901. doi: 10.1161/01.cir.101.25.2896 [Crossref]
- Koch DW, Leuenberger UA, Proctor DN, Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. Physiol 2003;551(1):337-44. doi: 10.1113/jphysiol.2003.042747 [Crossref]
- Lakatta EG.
Cardiovascular regulatory mechanisms in advanced age. Physiol
Rev 1993;73(2):413-67. doi: 10.1152/physrev.1993.73.2.413 [Crossref]
- Bassareo PP, Antonio Crisafulli A. Gender differences in hemodynamic regulation
and cardiovascular adaptations to dynamic exercise. Curr
Cardiol Rev 2020;16(1):65-72. doi: 10.2174/1573403X15666190321141856 [Crossref]
- Maruf FA, Ogochukwu UN, Dim PA, Alada R. Absence of sex differences in systolic blood
pressure and heart rate responses to exercise in healthy young adults. Niger J Physiol Sci [Internet] 2012[cited 2022 March
4];27(1):95-100. https://pubmed.ncbi.nlm.nih.gov/23235315/
- Pivarnik JM, Marichal CJ, Spillman T, Morrow JR Jr. Menstrual cycle phase affects temperature regulation during endurance exercise. J Appl Physiol 1992;72:543-5. doi: 10.1152/jappl.1992.72.2.543 [Crossref]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 1974;54(1):75-159. doi: 10.1152/physrev.1974.54.1.75 [Crossref]
