Rev Bras Fisiol Exerc 2022;21(4):217-31
doi: 10.33233/rbfex.v21i4.5247
ORIGINAL ARTICLE
Blood pressure response to dynamic resistance exercise with different
times under blood flow restriction on normotensive subjects: a randomized
crossover trial
Resposta da pressão
arterial ao exercício resistido com diferentes tempos sob restrição do fluxo
sanguíneo em indivíduos normotensos: um estudo randomizado cruzado
Leandro Lima de Sousa1,
Dahan da Cunha Nascimento1, Nicholas Rolnick2, Jessica Mycaelle da Silva Barbosa1, Robson Conceição
Silva1, Bruno Viana Rosa1, Thailson
Fernandes da Silva1, Carlos Ernesto Santos Ferreira1
1Universidade Católica de Brasília, DF, Brazil
2Lehman College,
Nova York, NY, Estados Unidos
Received: August 22, 2022; Accepted: August 30, 2022.
Correspondence: Leandro Lima de Sousa, leandrolsousa08@gmail.com
Sousa LL,
Nascimento DC, Rolnick N, Barbosa JMS, Silva RC, Rosa
BV, Silva TF, Ferreira CES. Blood pressure response
to dynamic resistance exercise with different times under blood flow
restriction on normotensive subjects: a randomized crossover trial. Rev Bras Fisiol Exerc 2022;21(4):217-31. doi: 10.33233/rbfex.v21i4.5247
Abstract
Introduction: Recommendations for time under blood flow restriction
(BFR) during resistance training (RT) vary between 5 to 10 minutes, and
beneficial effects on muscle mass and strength have already been reported.
However, there exists the potential for longer times under restriction to
produce greater acute activation of the exercise pressor reflex and subsequent
sympathetic pathways leading to a greater hemodynamic response. Objective:
To verify blood pressure responses to dynamic resistance exercise with
different times (5 vs. 10 minutes) under blood flow restriction in normotensive
subjects. Methods: In a randomized crossover trial design, twelve healthy
and physically active male participants completed a training with BFR under the
following protocols: control, BFR-5 minutes, BFR-10 minutes. Systolic blood pressure
(SBP) and diastolic blood pressure (DBP) measurements were taken by an
experienced researcher immediately after each exercise set. Results:
Both BFR-5 minutes and BFR-10 minutes induced acute elevations in SBP, DBP and
heart rate (HR) as the sets progressed, without statistical differences between
them. However, BFR-10 displayed a superior effect size for SBP and DBP compared
to BFR-5 minutes. Conclusion: Based on the results of this study,
the time under BFR during resistance exercise does not affect blood pressure
response in normotensive subjects.
Keywords: resistance training; blood flow restriction therapy,
blood pressure.
Resumo
Introdução: A recomendação de tempo sob restrição
de fluxo sanguíneo (RFS) durante o treinamento resistido (TR) pode variar entre
5 e 10 minutos, e já foram relatados efeitos benéficos para o desenvolvimento
da hipertrofia e força muscular. No entanto, existe o potencial que o longo
tempo sob restrição possa induzir maior ativação aguda do reflexo pressor
durante o exercício e subsequentemente das vias simpáticas levando a uma maior
resposta hemodinâmica. Objetivo: Verificar as respostas da pressão
arterial ao exercício resistido com diferentes tempos sob restrição de fluxo
sanguíneo em indivíduos normotensos. Métodos: Nesse estudo
randomizado cruzado, doze participantes do sexo masculino saudáveis e
fisicamente ativos completaram em ordem aleatória os seguintes protocolos:
controle, RFS-5 minutos e RFS-10 minutos. As medidas da pressão arterial
sistólica (PAS) e da pressão arterial diastólica (PAD) foram mensuradas por um
pesquisador experiente imediatamente após cada série do exercício. Resultados:
Tanto o RFS-5 minutos quanto o RFS-10 minutos induziram elevações agudas na
PAS, PAD e frequência cardíaca (FC) à medida que as séries progrediam, sem
diferenças estatísticas entre elas. No entanto, um tamanho efeito superior para
a PAS e PAD foi apresentado para a condição RFS-10 comparado a condição
RFS-5. Conclusão: Com base nos resultados do presente estudo, o
tempo de restrição do fluxo sanguíneo durante o exercício resistido não altera
a resposta pressórica em indivíduos normotensos.
Palavras-chave: treinamento de força; terapia de
restrição de fluxo sanguíneo; pressão sanguínea.
Introduction
Exercise training with blood flow restriction (BFR) is
considered a progressive clinical rehabilitation modality to improve muscle
mass and strength in patients that have musculoskeletal weakness in the process
of returning to heavy-load exercise [1]. Studies report comparable increases in
muscle mass compared to heavy-load resistance training (RT) [2,3], regardless
of absolute occlusion pressure, cuff width, and occlusion pressure prescription
method [3].
Despite the beneficial effect of BFR on lean mass and
muscle strength [1], there exists significant heterogeneity in the application
of potentially important BFR variables (e.g., absolute occlusion pressure, cuff
width, and occlusion pressure prescription method). When not properly applied
according to established guidelines, BFR may represent a safety concern and not
be suitable for clinical populations that may require more precise control of
BFR stimulus. Furthermore, a previous study stated that misuse of this method
could lead to acute and abnormal elevations in sympathetic activity and risk of
cardiovascular-related events (e.g., cardiac arrhythmia, myocardial infarction,
stroke and sudden cardiac death) [4].
One of the concerns of BFR training is its safety
profile for hypertensive and cardiovascular patients. In hypertensive
populations, the increase in systolic and diastolic blood pressure during BFR
training is higher than traditional exercise compared to normotensive peers
[5]. Also, diastolic blood pressure during BFR training is higher when compared
to traditional exercise [5]. Consequently, exercise demands on the
cardiovascular system approach or exceed free-flow high-intensity exercise [6].
Thus, despite the assertions of BFR safety, possible side effects should be
considered before the application in individuals with hypertension and
cardiovascular disease [4]. Importantly, acute- and longitudinal BFR studies in
patients with cardiovascular disease patients are poorly available [7].
A previous meta-analysis examining the effects of BFR
training on blood pressure stated that the included studies were not designed
to address whether BFR training affects blood pressure specifically and called
for research on this topic [8]. Moreover, considering the different BFR
application variables that may impact hemodynamic response, time under BFR is
interestingly not debated [9]. Time under BFR might affect chemical and
mechanical stimuli, activating the exercise pressor reflex and enhancing
sympathetic output while reducing parasympathetic activity [4]. Other studies
have sought to determine whether the continuous application of pressure could
alter physiologic responses such as metabolic stress [10]. Thus, there is
theoretical reason that manipulating time under BFR during RT might affect
acute physiological responses.
Traditionally, the occlusive stimulus during BFR is
applied continuously during exercise and the rest intervals (between 5 to 10
minutes total time under occlusion) [11]. There exists the potential for longer
times under restriction to produce greater acute activation of the exercise
pressor reflex and subsequent sympathetic pathways leading to a greater
hemodynamic response. However, no studies have yet focused on whether time
under restriction is an important variable in mediating the hemodynamic
response to BFR exercise.
Therefore, the purpose of this study was to verify if
the time of blood flow restriction alters blood pressure response during
resistance exercise in healthy individuals. We hypothesized that BFR training
with a longer time of restriction would display a higher hemodynamic response
than shorter time restriction.
Methods
This randomized crossover study was approved by the
Ethics and Research Committee of the Catholic University of Brasília, CAAE
39652920.4.0000.0029 and was conducted in accordance with the Declaration of
Helsinki. Twelve healthy and physically active (according to PAR-Q short
version) males [12], but inexperienced in resistance exercise, were recruited
for the study. All participants were informed about the purpose, practical
details, and possible risks associated with the experiment and before data
collections began, each gave their consent by signing a consent form. Exclusion
criteria were participants with any of the following conditions:
musculoskeletal injuries in the lower limbs, continuous use of medication and
nutritional supplements that could affect blood pressure response, resting
blood pressure ≥
140 x 90 mmHg, existing heart disease, peripheral vascular disease, diabetes,
BMI ≥ 30, and one
or more risk factors for thromboembolism [13].
Blood flow restriction protocols
This crossover trial was conducted within five visits,
at the same time of day, separated at least 72 and no more than 96 hours. Signs
of swelling and shortness of breath, changes in skin temperature, presence of
tachycardia, pain or discoloration and swollen or distended varicose veins were
visually monitored [14]. They were instructed not to perform any exercise 72-96
hours before exercise protocol.
The first visit consisted of signing the Informed
Consent Form, completing questionnaires to assess the level of physical
activity (IPAQ - short version), physical and health condition (PAR-Q), risk
stratification for thromboembolism [13], and screening for medications and food
supplements that could affect blood pressure. In addition, patients were
evaluated by an experienced cardiologist. First, participants rested for 10
minutes in the supine position, relaxed, head and heels supported in a room
with comfortable temperature (~25°C). Then, resting blood pressure was measured
using an automatic monitor (Microlife, Shenzhen,
China) where a cuff was placed on the participant’s left arm, approximately 2
cm above the cubital fossa. Right after, a 12-lead electrocardiogram followed
by an ankle-brachial blood pressure index (ABI) test was performed to verify
the existence of peripheral vascular disease [15]. Body composition was
evaluated by Dual-energy X-ray Absorptiometry (DXA). Calibration of equipment
was provided and phantom was used to check calibration daily before body
composition evaluation. The tests included a complete body scan in the supine
position with the apparatus calibrated and operated by a technically trained
professional. All metal objects were removed from the participant before the
scan.
Finally, 1-RM was also evaluated in the first session.
The test started with five minutes of general warm-up performed on a treadmill
(Imbrasport Millenium ATL, Imbramed, Porto Alegre, Brazil) at ≤ 85% heart rate reserve. Afterward,
participants performed three static stretching exercises for the hamstrings,
hips, and quadriceps (1 set of 10-s). After that, the participants were
positioned in the 45-degree leg press (PowerTech, Riguetto, Campinas, Brazil), maintaining the alignment of
the ankle, knee, and hip joints to perform the specific warm-up and the test
itself [16]. The 1-RM was found in a maximum of five attempts (separated by 3
minutes of recovery for each attempt). During the eccentric phase, the
individuals were instructed to bend the knees to 90° flexion and in the
concentric phase, to almost complete extension (approximately 20° of knee
flexion). To have greater precision in the result, 1-RM was re-tested 96 hours
after with a similar procedure, but the first load attempted was the load found
in session one. For the value of 1 RM found, the weight of the leg press
platform (which had 40 kg) was considered. During all tests, at least two
researchers provided support to minimize the occurrence of exercise-related
accidents.
As mentioned, the second session served as a retest of
the 1-RM. In addition, this session served as familiarization to low-load BFR
exercise as each participant performed one set of 30 repetitions at 20% 1RM
using 50% arterial occlusion pressure determined in the 45-degree leg press.
In visits 3, 4, and 5, the exercise protocol sessions
were carried out in one of the three conditions (control, BFR-5 minutes, BFR-10
minutes) described below. Interventions were conducted in the crossover model
and subjects were assigned to conditions by randomly picking a protocol inside
of an envelope. For the study scheme, see Figure 1.
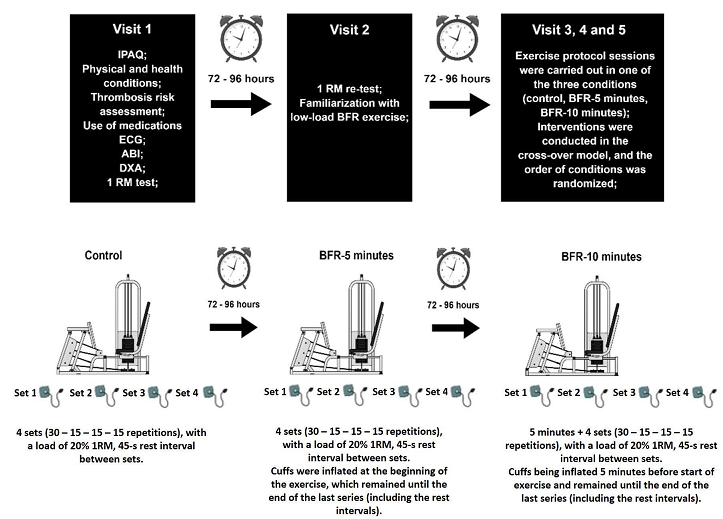
IPAQ = International Physical Activity Questionnaire;
PAR-Q = Physical Activity Readiness Questionnaire; ECG = electrocardiogram; ABI
= ankle brachial index; DXA = Dual-energy X-ray absorptiometry; 1 RM = 1
repetition maximum; BFR = blood flow restriction; BFR-5 = blood flow
restriction 5 minutes protocol; BFR-10 = blood flow restriction 10 minutes
protocol; AOP = arterial occlusion pressure. N = 12 participants
Figure 1 - Study scheme. General visits details
Exercise protocol
The exercise was performed in the 45-degree leg press
(PowerTech, Riguetto,
Campinas, Brazil) that consisted of 4 sets (30 – 15 – 15 – 15 repetitions) with
a load of 20% 1RM, ~45-s rest interval between sets, and a rhythm of 1-s for
concentric and 1-s for eccentric (controlled by an audible metronome); thus,
the exercise duration was approximately 5 minutes. Participants were
comfortably positioned on the equipment and instructed to maintain the
alignment between ankles, knees, and hips. In addition, a researcher controlled
the range of motion (90° degrees of knee flexion in the eccentric phase and
almost complete extension - approximately 20° degrees flexion - during the
concentric phase). All participants performed the exercise under three
conditions:
In protocol 1 (control), exercise was performed
without BFR. In protocol 2 (BFR-5 minutes), cuffs were inflated at the
beginning of the exercise and remained inflated until the end of the last
series (including the rest intervals). Thus, duration under BFR was equal to
the exercise duration (5 minutes). In protocol 3 (BFR-10 minutes), the same
actions as in protocol 2 (BFR-5 minutes) were performed, but to maintain the
same exercise volume as control and BFR-5 minutes, the cuffs were inflated for
5-minutes prior to beginning the leg press exercise making the duration under
BFR 10 minutes (5 minutes previous + 5-minutes of exercise). The interval between
protocols was a minimum of 72 and a maximum of 96 hours and participants
reported to the lab at similar times of day to minimize diurnal variations
In protocols BFR-5 minutes and BFR-10 minutes, BFR was
induced by a pair of inflatable cuffs (Premium, Zhejiang, China) with 20 cm
width x 42 cm length (cuff bladder = 17 cm width x 37 cm length) placed on the
proximal part of the thighs (as close as possible to the inguinal crease) with
50% of the total arterial occlusion pressure (AOP).
Arterial Occlusion Pressure (AOP)
Because of hemodynamic variations, AOP was checked
before performing each exercise protocol (BFR-5 minutes and BFR-10 minutes).
With the volunteer seated on the 45-degree leg press, two measurements were
taken on each leg (one in recovery position and other in exercise execution
position – feet on the platform) (Figure 2). A pair of inflatable cuffs
(Premium, Zhejiang, China) was placed on the proximal part of the thighs (as
close as possible to the inguinal crease). A small amount of water-based
conductive gel (Mercur, Santa Cruz do Sul, RS,
Brazil) was placed in the portable vascular doppler probe (DV 610B, Medmega, Franca, SP, Brazil) and this was positioned
perpendicular on the dorsalis pedis artery with minimal pressure. AOP was
determined when arterial pulse was interrupted according to previous studies
[17,18]. Values of pressure used on cuffs are described in Table II.

A = resting position; B = exercise position. N = 12
participants
Figure 2 - Legs positions during arterial occlusion
pressure measurements
Blood pressure measurement
Measurements were taken by an experienced researcher
immediately after each exercise set. Furthermore, a cuff size corresponding to
the participant’s arm size was used [19]. A blood pressure cuff (Welchallyn, Chicago, IL, USA) was placed on the
participant’s left arm, approximately 2 cm above the cubital fossa. A
researcher supported the participant’s arm on a support so that the participant
remained totally relaxed and the cuff was inflated 10 mmHg above Korotkoff
sound stopped. Thus, cuff was deflated slowly and auscultatory measurement of
systolic (SBP) and diastolic (DBP) blood pressure was performed (SBP and DBP)
was annotated when the Korotkoff sound started and stopped, respectively) [20].
To not interfere in the time duration under BFR, measurements at post set 4
were taken after cuffs were deflated. Additionally, participants were also
advised to maintain an empty bladder and not to talk during protocols (control,
BFR-5 minutes, BFR-10 minutes) as these variables may impact blood pressure
reading [19,20,21].
Heart rate monitoring during exercise
Heart rate (HR) was measured using Polar’s FT1 HR
monitor system (Polar, Kempele, FI) via a chest-worn
sensor strap and a wristwatch HR receiver unit. To improve skin contact, a
small amount of water-based conductive gel (Mercur,
Santa Cruz do Sul, RS, Brazil) was placed in the sensor.
Statistical analysis
A two-way repeated measures ANOVA was conducted to
examine the effects of different restriction times on blood pressure responses.
Data are presented in mean ± standard deviation, unless otherwise stated.
Analysis of the studentized residuals showed that there was normality as
assessed by the Shapiro-Wilk test of normality and no outliers as assessed by
no studentized residuals greater than ± 3 standard deviations. When a
significant interaction was observed, a simple main effects analysis was applied
and a Bonferroni Post-hoc was applied. For the two-way repeated measures ANOVA
statistical test, the intragroup effect size was calculated for the variables
SBP, DBP and HR. The omega squared (Ω2) recommended for small samples was used and
values ≤ 0.01, 0.01
– 0.06, 0.06 – 0.14 and > 0.14 were considered: trivial, small, medium, and
large, respectively [22]. Also, a delta (Δ) analysis was performed, which was calculated as follows: Δa =
set 1 minus pre-training; Δb =
set 2 minus pre-training; Δc =
set 3 minus pre-training; Δd =
post-training minus pre-training and post-training. Cohen’s d was used to
effect size between moments pre-exercise and set 1, pre-exercise and set 2,
pre-exercise and set 3 and pre-exercise and post-training for variables SBP,
DBP and HR. Hence, One-way ANOVA was conducted for comparisons between Δ group differences.
Coefficient of variation (CV) was used to calculate
within participant variation (CV% = [SD/mean] x 100). The CV for leg press was
17.44%. Considering a minimum difference of 10 mmHg for DBP between groups
[23,24], the power observed for interaction between restriction time and time
on DBP was 0.85, effect size of 1.16, with an alpha error probability of 0.01.
Power was calculated using G*Power 3.1.6 [25]. An alpha level of α ≤ .05 was
considered significant, and all calculations were performed using SPSS (version
20.0).
Results
No adverse events occurred, and all participants were
able to complete each exercise intervention. Intraclass correlation coefficient
between 1-RM test and re-test was ICC = 0.92.
The characteristics of the sample are displayed in
Table I. Table II reports mean pressure applied to the participant’s thighs (in
mmHg).
Table I - Characteristics of participants. Values described
as mean ± standard deviation
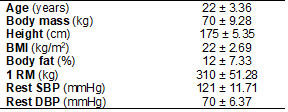
BMI = body mass index; 1 RM = 1 repetition maximum;
SBP = systolic blood pressure; DBP = diastolic blood pressure. N = 12
participants
Table II - Pressure used on cuffs during BFR protocols.
Values described as mean ± standard deviation

BFR = blood flow restriction; BFR-5 = 5-minute
protocol; BFR-10 = 10-minute protocol. n= 12 participants
There was no interaction between time under
restriction and moments on SBP, F(8, 88) =
1.88, p = 0.07. However, a main effect of time was observed F(4, 44) = 27.83, p = 0.001. As shown at table
III, compared to pre-training, SBP was higher at sets one, two and three only
for BFR conditions (mean difference of 19.50; 17.66 mmHg, 26.00; 28.50 mmHg,
26.16; 29.83 mmHg for BFR-5 minutes; BFR-10 minutes respectively), as well as,
only for post-training at BFR-10 minutes (mean difference of 15.00 mmHg).
Also, there was an interaction between time under
restriction and moments on DBP, F(8,88) = 8,86, p =
0.001. For BFR-5 minutes, a statistically higher DBP at set three was observed
compared to pre-training (mean difference of 8.75 mmHg). Besides, a
statistically lower DBP at post-training was observed for BFR-10 minutes when
compared to pre-training (mean difference of -11.41 mmHg). In addition, a
statistically higher DBP was observed for sets one, two and three for BFR
conditions compared to control (mean difference of 10.00; 10.00 mmHg, 11.66;
15.00 mmHg, 15.83; 15.83 mmHg for BFR-5 minutes; BFR-10 minutes respectively).
See Table III.
There was no interaction between time under
restriction and moments on HR, F(8, 88) =
0.89, p = 0.58. However, a main effect of time was observed F(4, 44) = 75.24, p = 0.001. For BFR condition, a
statistically higher HR at sets one, two, three and post-training compared to
pre-training was observed (mean difference of 20.66; 22.00 bpm, 23.08; 27.25
bpm, 25.08; 29.00 bpm and 26.58; 30.08 bpm for BFR-5 minutes; BFR-10 minutes
respectively). Finally, for control session, a statistically higher HR at sets
one, two, three and post-training compared to pre-training was observed (mean
difference of 27.16 bpm, 27.83 bpm; 30.50 bpm and 32.75 bpm respectively). See
Table III.
Table III - Blood pressure and heart rate response between
protocols
For delta analysis, no differences between groups and
moments for SBP was observed. However, DBP values were statistically higher for
BFR conditions at moments post set 1, post set 2 and post set 3 compared to
control. Thus, adding BFR demonstrated a superior increase in DBP, regardless
of the duration used.
Effect size
Considering the effect size values between groups for
main effect of time, a superior magnitude of treatment effect for BFR-10
minutes as compared with control and BFR-5 minutes were observed for SBP, and
DBP. For HR, no differences between BFR-10 minutes and Control were observed,
but a higher effect size for BFR-10 minutes compared to control was observed.
See Table IV.
Table IV - Values of effect size for main effect of time
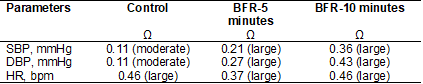
Ω = effect size; SBP = systolic blood pressure; DBP =
diastolic blood pressure; HR = heart rate; BFR = blood flow restriction. N = 12
participants. Source: authors
Discussion
To our knowledge, this is the first study to examine
the hemodynamic response in normotensive participants after different times
under blood flow restriction. Therefore, the significant new findings are 1)
Both BFR-5 minutes and BFR-10 minutes induced acute elevations in SBP, DBP, and
HR as the sets progressed, without differences between them. 2) Furthermore,
DBP demonstrated a superior increase with BFR exercises compared to control
group, regardless of the time used. This indicates that the duration of BFR up
to 10 minutes does not alter pressure responses in normotensive subjects.
Although studies to make similar comparisons in
normotensive individuals are scarce, a previous acute study demonstrated that
BFR training (20% 1RM) in hypertensive women subjects provoked increases SBP
and DBP similar to high-load RT (65% 1RM) in the leg press exercise, with
additional increases in blood pressure observed during the rest intervals
compared to pre-exercise resting values [26]. The protocol consisted of three
sets of 15 repetitions with 30 seconds rest with a continuously applied cuff
pressure throughout the three sets [26,27]. Thus, it is possible to infer that
time under BFR totaled between 4 to 6 minutes. During exercise in the BFR
condition, SBP and DBP elevated to 237 mmHg and 139 mmHg, generating a larger
hemodynamic response than traditional strength training while also displaying
greater values of blood pressure during the rest intervals (e.g., during 2nd
rest interval - SBP = 182 mm Hg vs. 143 mmHg in high load RT, p < 0.05).
Similar results in hypertensive patients were observed
in another intervention [28]. Greater acute increases in SBP (212 mmHg) and DBP
(123 mmHg) similar to high-load RT were recorded along with greater relative
increases in blood pressure values during the pauses between sets. The cuff
pressure was sustained during the experimental sessions of BFR and released
immediately after the end of the third set [26,27]. While not reported, we
estimate based on repetition cadence that time under BFR was between 4 to 6
minutes. Taken together, these results might shape guidance that hypertensive
participants may benefit from deflation of the BFR stimulus (e.g., intermittent
BFR) at some point during BFR exercise, as that could attenuate increases in
SBP and DBP [29] observed during the pauses.
A previous study showed potential applicability of a
cyclical BFR protocol and its effect on blood pressure and norepinephrine
levels compared to conventional RT [29]. The exercise session duration for both
conditions was 40 minutes (divided into 4 x 10 min blocks). For BFR training,
each 10-min block consisted of a 5-min exercise period with the cuff inflated
and 5-min reperfusion with the cuff deflated. For conventional RT (65% of 1RM),
the session was performed in the same manner but without inflatable thigh cuffs
[29]. Results demonstrated that plasma norepinephrine, stroke volume, cardiac
output, mean arterial pressure, and total peripheral resistance were augmented
with conventional RT compared to BFR training [29]. This attenuated increase in
sympathetic activity and hemodynamic responses during cyclical BFR (5-min
exercise period with the cuff inflated and 5-min reperfusion with the cuff
deflated) could be potentially adapted for clinical populations [29].
However, contrary to research on hypertensive
populations, our data did not show differences between different times under
BFR in hemodynamic response. Importantly, the hemodynamic response to BFR is
less exaggerated in normotensive populations [5]. For this reason, these data
should not be extrapolated to populations where excessive blood pressure
elevations during exercise may be a concern. Thus, studies investigating the
time under BFR in specific populations (e.g., hypertensive patients) should be
carried out to better determine the parameters for prescribing this type of
exercise.
A recent guideline recommended that BFR RT restriction
time should be between 5 to 10 minutes per exercise with at least 1-3 minutes
of reperfusion between exercises [11]. Conversely, for AT the restriction time
recommended is 5 to 20 minutes [11]. However, the increase in time under
restriction during AT may unnecessarily increase hemodynamic responses,
particularly in clinical patients whose pressor reflex may be altered. Although
metabolic accumulations are typically much less in AT allowing for longer times
under restriction.
Future investigations into BFR AT exercise could look
to incorporate a similar model as the current study to determine differential
hemodynamic responses in BFR AT protocols of different time intervals.
The literature is limited with regards to protocols
directly comparing BFR AT to BFR RT on hemodynamics. A previous study compared
the effects of BFR-RT (4 sets x 15 – 15 – 15 – 15 at 30% of 1RM at 50% AOP,
with 1 min interval between sets) with BFR AT exercise (composed of 20 minutes
of continuous treadmill walking at 40% of VO2peak with 50% AOP) on hemodynamic
responses in older adults [30]. In both sessions, continuous application of
cuff pressure was maintained throughout the exercise, being released just after
the last repetition of the last set during BFR protocol and at 20 min during
aerobic exercise with BFR. Interestingly, independently of a longer time
restriction with AT, a lower SBP, DBP, peripheral vascular resistance peak and
a faster heart rate recovery was observed compared to BFR-RT [30]. Similar
results of a lowering of SBP and DBP were observed in normotensive subjects
when BFR-RT was compared to BFR AT in another study [31]. These results raise
important considerations with BFR AT that may impact prescription of BFR RT in
clinical practice. Despite the longer time under restriction, BFR AT appears to
be a suitable strategy to mitigate the excessive increases in SBP and DBP
associated with BFR RT.
While more research is needed to determine optimal
application parameters (e.g., intensity, duration and BFR pressures), BFR AT
likely displays these changes due to an attenuated accumulation of intramuscular
metabolites, reducing the magnitude of the exercise pressor reflex and
subsequent sympathetic activation despite the longer time under restriction
[32]. Therefore, based on the results of our study, future research should
investigate whether the addition of passive restriction prior to a bout of BFR
AT could further alter hemodynamic responses. Furthermore, heart rate
variability has considerable potential to assess the effects of time under
restriction in autonomic nervous system in health and cardiovascular patients
and warrants further research.
Finally, the increase in blood pressure during
exercise occurs by a mechanism known as the pressor reflex, in which it
stimulates the sympathetic nervous system and inhibits the parasympathetic
nervous system [4]. In our results, SBP did not present a significant
difference between protocols, however the DBP was significantly higher in the
protocols with BFR regardless of time under BFR. We speculate that the increase
in DBP in BFR-5 and BFR-10 is due to the venous system congestion caused by the
application of cuffs during exercise [5].
Some limitations of the present study should be
highlighted. Cross-over designs may face problems with carryover effects and
possible systematic differences between hemodynamic responses during the later
compared to the earlier sessions. Also, the indirect cuff method used to
measure blood pressure response during BFR training might underestimate SBP and
overestimate DBP values and the validity is very poor when compared to that of
directly measured intra-arterial pressure [33]. However, considering the
practical applicability, auscultatory technique is still the traditional
approach for measuring SBP and DBP in clinical settings. Finally, blood
pressure was measured after and not during exercise and post-training was
measured after deflation of the cuff to maintain similar times under
restriction, so the values shown may differ from those achieved during
exercise. In alignment with our methodology, some papers measured blood
pressure after cuff was deflated [26,34]. Further, there may be an
underestimation of the hemodynamic changes post-exercise due to the deflation
of the cuff. That’s why only set three was used for delta analysis and not set
4 (post-training). Thus, the increased BP in earlier sets was attenuated by the
deflation. This in fact underestimated the BP response during our protocol.
Future studies should maintain the restriction while obtaining blood pressure
values as that may give a more accurate assessment.
Practical applications
While both long and short time under BFR can
potentially increase blood pressure during exercise, long time (10 minutes)
under BFR displayed a superior effect size for SBP and DBP in normotensive
individuals. Although speculative, manipulating BFR variables strategically
could increase the safety of medically compromised populations (e.g.,
hypertensive individuals and patients under cardiac rehabilitation). This could
increase the number of hypertensive individuals who pursue BFR training as a
mode of exercise.
Conclusion
Based on the present study results, time under BFR
during resistance exercise does not affect blood pressure response in
normotensive subjects despite a larger effect size in longer durations. However,
due to the overall lack of studies in this thematic, future research on this
topic is warranted in upper body RT as well as in hypertensive populations.
Academic link
This article represents part of Leandro Lima de
Sousa's master's thesis, supervised by the professor Dr. Carlos Ernesto Santos
Ferreira from the Catholic University of Brasília, Taguatinga, Distrito
Federal, Brazil.
Conflict of interest
NR is the founder of THE BFR PROS, a BFR education
company that provides BFR training workshops to fitness and rehabilitation
professionals across the world using a variety of BFR devices. NR has no
financial relationships with any cuff manufacturers/distributors. The remaining
authors declare that the research was conducted in the absence of any
commercial or financial relationships that could be construed as a potential
conflict of interest.
Funding source
The present study was carried
out without funding.
Authors’ contributions
Research conception and
design: Sousa LL, Ferreira CES, Nascimento DC; Data
collection: Sousa LL, Silva RC, Silva TF, Barbosa JMS; Data analysis and
interpretation: Nascimento DC, Rolnick N, Rosa BV; Statistical analysis:
Nascimento DC, Rolnick N; Writing of the manuscript: Nascimento DC, Rolnick N,
Sousa LL, Rosa BV, Ferreira CES; Critical review of the manuscript for
important intellectual content: Nascimento DC, Rolnick N, Ferreira CES.
- Hughes L, Paton B, Rosenblatt B, Gissane C,
Patterson SD. Blood flow restriction training in clinical musculoskeletal
rehabilitation: a systematic review and meta-analysis. Br J Sports Med
2017;51(13):1003-11. doi: 10.1136/bjsports-2016-097071 [Crossref]
- Centner C, Wiegel P, Gollhofer A, Konig D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med 2019;49(1):95-108. doi: 10.1007/s40279-018-0994-1 [Crossref]
- Lixandrão ME, Ugrinowitsch C, Berton R, Vechin FC, Conceição MS, Damas F, et al. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med 2018;48(2):361-78. doi: 10.1007/s40279-017-0795-y [Crossref]
- Spranger MD, Krishnan AC, Levy PD, O'Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol 2015;309(9):H1440-1452. doi: 10.1152/ajpheart.00208.2015 [Crossref]
- Domingos E, Polito MD. Blood pressure response between resistance exercise with and without blood flow restriction: A systematic review and meta-analysis. Life Sci 2018;209:122-31. doi: 10.1016/j.lfs.2018.08.006 [Crossref]
- Manini TM, Clark BC. Blood flow restricted exercise and skeletal muscle health. Exerc Sport Sci Rev 2009;37(2):78-85. doi: 10.1097/JES.0b013e31819c2e5c [Crossref]
- Cuyul-Vasquez I, Leiva-Sepulveda A, Catalan-Medalla O, Berrios-Contreras L. Blood flow restriction training for people with cardiovascular disease: An exploratory review. Rehabilitacion (Madr) 2020;54(2):116-27. doi: 10.1016/j.rh.2020.01.005 [Crossref]
- Wong V, Song JS, Bell ZW, Yamada Y, Spitz RW, Abe T, et al. Blood flow restriction training on resting blood pressure and heart rate: a meta-analysis of the available literature. J Hum Hypertens 2021. doi: 10.1038/s41371-021-00561-0 [Crossref]
- Nascimento DC, Schoenfeld BJ, Prestes J. Potential implications of blood flow restriction exercise on vascular health: a brief review. Sports Med 2020;50(1):73-81. doi: 10.1007/s40279-019-01196-5 [Crossref]
- Suga T, Okita K, Takada S, Omokawa M, Kadoguchi Yokota T, et al. Effect of multiple set on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. Eur J Appl Physiol 2012;112(11):3915-20. doi: 10.1007/s00421-012-2377-x [Crossref]
- Patterson SD, Hughes L, Warmington S, et al. Blood flow restriction exercise: considerations of methodology, application, and safety. Front Physiol 2019;10:533. doi: 10.3389/fphys.2019.00533 [Crossref]
- Chisholm DM, Collis ML, Kulak LL, Davenport W, Gruber N. Physical
activity readiness. B C Med J 1975;17:375-8.
- Motykie GD, Zebala LP, Caprini JA, Lee CE, Arcelus JI, Reyna JJ, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis 2000;9(3):253-62. doi: 10.1023/a:1018770712660 [Crossref]
- O'Brien A, Redley B, Wood B, Botti M, Hutchinson AF. STOPDVTs: Development and testing of a clinical assessment tool to guide nursing assessment of postoperative patients for Deep Vein Thrombosis. J Clin Nurs 2018;27(9-10):1803-11. doi: 10.1111/jocn.14329 [Crossref]
- Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126(24):2890-2909. doi: 10.1161/CIR.0b013e318276fbcb [Crossref]
- Brown LE, Weir JP. ASEP procedures recommendation I: accurate assessment
of muscular strength and power. J Exerc Physiol [Internet]. 2001[cited 2022 May 12];4(3). Available
from: https://www.asep.org/asep/asep/Brown2.pdf
- Laurentino GC, Ugrinowitsch C, Roschel H, et al. Strength training with blood flow restriction diminishes myostatin gene expression. Med Sci Sports Exerc. 2012; 44(3):406-12. doi: 10.1249/MSS.0b013e318233b4bc [Crossref]
- Moriggi R Junior, Mauro HD, Dias SC, Matos JM, Urtado MB, Camarço NF, et al. Similar hypotensive responses to resistance exercise with and without blood flow restriction. Biol Sport 2015;32(4):289-94. doi: 10.5604/20831862.1163691 [Crossref]
- Muntner P, Shimbo D, Carey RM, Charleston TG, Misra S, Meyers MG, et al. Measurement of blood pressure in humans: a scientific statement from the american heart association. Hypertension 2019;73(5):e35-e66. doi: 10.1161/HYP.0000000000000087 [Crossref]
- Vischer AS, Burkard T. Principles of blood pressure measurement - current techniques, office vs ambulatory blood pressure measurement. Adv Exp Med Biol 2017;956:85-96. doi: 10.1007/5584_2016_49 [Crossref]
- Severin R, Sabbahi A, Albarrati A, Phillips SA, Arena S. Blood pressure screening by outpatient physical therapists: a call to action and clinical recommendations. Phys Ther 2020;100(6):1008-19. doi: 10.1093/ptj/pzaa034 [Crossref]
- Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med 2021;31(1):010502. doi: 10.11613/BM.2021.010502 [Crossref]
- Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 1995;155(7):701-9. https://pubmed.ncbi.nlm.nih.gov/7695458/
- Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens 2003;12(3):293-7. doi: 10.1097/00041552-200305000-00011 [Crossref]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39(2):175-91. doi: 10.3758/bf03193146 [Crossref]
- Pinto RR, Polito MD. Haemodynamic responses during resistance exercise with blood flow restriction in hypertensive subjects. Clin Physiol Funct Imaging 2016;36(5):407-13. doi: 10.1111/cpf.12245 [Crossref]
- Poton R, Polito MD. Hemodynamic response to resistance exercise with and without blood flow restriction in healthy subjects. Clin Physiol Funct Imaging 2016;36(3):231-6. doi: 10.1111/cpf.12218 [Crossref]
- Pinto RR, Karabulut M, Poton R, Polito MD. Acute resistance exercise with blood flow restriction in elderly hypertensive women: haemodynamic, rating of perceived exertion and blood lactate. Clin Physiol Funct Imaging 2018;38(1):17-24. doi: 10.1111/cpf.12376 [Crossref]
- Sprick JD, Rickards CA. Cyclical blood flow restriction resistance exercise: a potential parallel to remote ischemic preconditioning? Am J Physiol Regul Integr Comp Physiol 2017;313(5):R507-R517. doi: 10.1152/ajpregu.00112.2017 [Crossref]
- Sardeli A, Ferreira M, Santos L, Cavaglieri C, Chacon-Mikahil MJS. Cardiovascular responses during and after aerobic and strength exercises with blood flow restriction in older adults. 2021. doi: 10.1016/j.scispo.2021.04.008 [Crossref]
- May AK, Brandner CR, Warmington SA. Hemodynamic responses are reduced with aerobic compared with resistance blood flow restriction exercise. Physiol Rep 2017;5(3). doi: 10.14814/phy2.13142 [Crossref]
- Loenneke JP, Thrower AD, Balapur A, Barnes JT, Pujol TJ. Blood flow-restricted walking does not result in an accumulation of metabolites. Clin Physiol Funct Imaging 2012;32(1):80-2. doi: 10.1111/j.1475-097X.2011.01059.x [Crossref]
- Dankel SJ, Kang M, Abe T, Loenneke JP. A meta-analysis to determine the validity of taking blood pressure using the indirect cuff method. Curr Hypertens Rep 2019;21(1):11. doi: 10.1007/s11906-019-0929-8 [Crossref]
- Araújo JP, Silva ED, Silva JC, Souza TS, Lima EO, Guerra I, Sousa MS. The acute effect of resistance exercise with blood flow restriction with hemodynamic variables on hypertensive subjects. J Hum Kinet 2014;12;43:79-85. doi: 10.2478/hukin-2014-0092 [Crossref]
