Rev Bras Fisiol Exerc 2022;21(4):286-94
doi: 10.33233/rbfex.v21i4.5379
REVIEW
Is there a benefit in unifying the concepts of sarcopenia and dynapenia in patients with sarcopenic obesity elective for
bariatric surgery? A conceptual review
Is there a benefit in unifying the concepts of sarcopenia and dynapenia in patients with sarcopenic obesity elective for
bariatric surgery? A
conceptual review
Luji Iseki
Takenami1, Ana Maria Sales Gomes Filha1, Eric Simas Bomfim1, Laura Souza Lagares1,
Rodrigo Colares de Macedo1, Luis Alberto
Bastos de Almeida1,2, Clarcson Plácido
Conceição dos Santos1
1Escola Bahiana
de Medicina e Saúde Pública, Salvador, BA, Brazil
2Universidade Estadual de Feira de
Santana, Feira de Santana, BA, Brazil
Received: April 13, 2022; Accepted:
June 30, 2022.
Correspondence: Clarcson
Plácido Conceição dos Santos, clarcson@hotmail.com
How to
cite
Takenami LI, Gomes Filha AMS,
Bomfim ES, Lagares LS, Macedo RC, Almeida LAB, Santos CPC. Is there a benefit in unifying the concepts of sarcopenia and dynapenia in patients with sarcopenic obesity elective for
bariatric surgery? A
conceptual review. Rev Bras
Fisiol Exerc 2022;21(4):286-94.
doi: 10.33233/rbfex.v21i4.5379
Abstract
Introduction: Sarcopenic obesity is a growing condition globally,
which can affect not only the elderly population but also the young population,
leading to a reduction in quality of life and predisposing the development of
other comorbidities. Methods: The present literature review revisited
the conceptual formation of sarcopenia and dynapenia,
investigated the physiological mechanisms of sarcopenic obesity, exploring the
benefits of bariatric surgery in this context. Results: The available
evidence of improvement in muscle strength even with a decrease in the amount
of lean mass in patients undergoing bariatric surgery exposes the scarcity of
studies regarding the association of metabolic factors with decreased muscle
strength. Conclusion: The reliability of the use of the terms dynapenia and sarcopenia as a cause-effect relationship is
questioned and further studies are needed.
Keywords: obesity; dynapenia;
sarcopenia; bariatric, surgery
Resumo
Introdução: A obesidade sarcopênica
é uma condição crescente globalmente, podendo acometer não somente a população
idosa como também a população jovem, gerando redução da qualidade de vida e
predispondo o desenvolvimento de outras comorbidades. Métodos: A
presente revisão de literatura revisitou a formação conceitual da sarcopenia e dinapenia, investigou os mecanismos fisiológicos da
obesidade sarcopênica e explorou os benefícios da
cirurgia bariátrica nesse contexto. Resultados: As evidências
disponíveis de melhoria na força muscular, mesmo tendo diminuição da quantidade
de massa muscular em pacientes submetidos à cirurgia bariátrica, expõe a
escassez de estudos referentes a associação de fatores metabólicos com a
diminuição de força muscular. Conclusão: Dessa forma, a confiabilidade
do uso dos termos dinapenia e sarcopenia enquanto
relação de causa-efeito é questionada e mais estudos são necessários para
investigar essa relação.
Palavras-chave: obesidade; dinapenia;
sarcopenia; bariátrica, cirurgia.
Introduction
Sarcopenic obesity is a condition determined by the
decrease in the amount of lean mass and muscle strength associated with the
increase in fat mass [1,2] and is defined by the existence of diagnoses of
sarcopenia and obesity.
The currently used concept of sarcopenia comprises the
decrease in muscle strength as an intrinsic process to the loss of lean mass
and is related to the aging process [2]. However, in the context of sarcopenic
obesity, this may not apply [3,4]. Studies with patients that undergo bariatric
surgery have demonstrated that despite the decrease in lean mass, it is
possible to obtain gains in muscle strength [5,6].
Bariatric surgery has been successful in the treatment
of obesity, being able to prevent the emergence of other comorbidities, also
improving quality of life and functional capacity. Although the literature is
wide on the causes of loss of lean mass and muscle strength associated with
aging, there is not much evidence regarding the influence of metabolic syndrome
on this same process [1].
The present study aims to revisit the construction of
the concepts of sarcopenia and dynapenia, investigate
the physiological mechanisms of sarcopenic obesity and explore what benefits
can be promoted by bariatric surgery.
Methods
To meet the purposes of this literature review, a
search for studies on sarcopenic obesity, and its association with bariatric
surgery, sarcopenia, and dynapenia was performed in
the databases: PubMed/Medline and Periódicos CAPES.
The minimum limit of the publication date of the studies was not proposed. The
search was completed in September 2022. The heterogeneity of the studies was
significant to the characteristics of the population, the study design, and the
analysis of the variables.
Results
In the studies of this review, a divergence of
concepts about sarcopenia was identified with that being used with comparative
purpose. Some risk factors for sarcopenia and dynapenia
were found and analyzed as part of a unique system.
Sarcopenia and dynapenia concepts
Table I describes the primitive concepts of sarcopenia
and dynapenia and compares them to the actual ones.
Table I – Sarcopenia and dynapenia
concepts

Muscular, neuromuscular, and metabolic factors
The Figure 1 presents some of the main risk factors related to elderly obese
patients as part of a unique system.
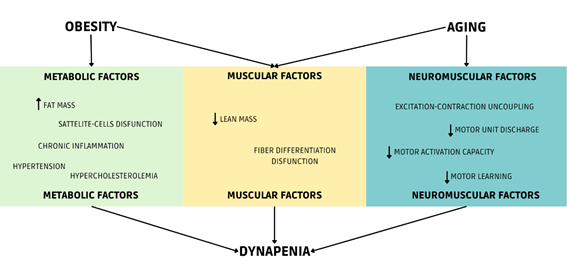
Source: Author himself
Figure 1 - Multifactorial system of dynapenia
in elderly obese patients after bariatric surgery
Discussion
Sarcopenia
The term sarcopenia, since its first use in the
literature, has undergone several changes in its concept and has not yet been
established [2]. Although it was previously associated with the process related
to aging [2,7], the most used concept today for sarcopenia is the association
of the processes of loss of lean mass and consequent loss of strength, with
loss of physical performance being the parameter of severity of this condition
[2].
The most commonly used diagnostic criteria for
sarcopenia are defined by the European Working Group on Sarcopenia in Older
People (EWGSOP) [2]. Due to the change in the concept of sarcopenia - in which
the loss of strength was considered an essential parameter for the diagnosis -
there was also an update of the diagnostic criteria used by the EWGSOP,
resulting in the EWGSOP2 [2]. The parameters for diagnosing sarcopenia
according to the EWGSOP2 are evaluated based on a priority order, with the
first parameter being muscle strength, the second being the amount of lean mass
and the third being physical performance [2]. Each of these has specific tests
and cutoff values that define sarcopenia [2].
The reduction of the first two parameters – muscular
strength and amount of lean mass below the cut-off values – indicate
sarcopenia, while the reduction of the third parameter – loss of physical
performance – suggests severe sarcopenia [2].
Dynapenia
The concept of dynapenia was
initially defined by Clark & Manini [8] as the age-related process of the
loss of muscular strength [7,9]. With the inclusion of loss of strength as a
parameter for sarcopenia, the term dynapenia was no
longer used [8].
Authors began to opt for the intuitive use of
sarcopenia as a term that describes the aging-related loss of both muscle
strength and strength of lean mass [10]. This unifying of concepts refers to
the existence of causality between sarcopenia and dynapenia,
influencing researchers to describe sarcopenia as responsible for the
occurrence of dynapenia [8]. Dynapenia,
however, can have several causes, not only related to loss of lean mass [9].
Sarcopenia vs dynapenia: prognosis and
physiological mechanisms
Although not predisposing to a direct risk to life, sarcopenia
is a comorbidity for other conditions of considerable morbidity and mortality,
such as falls from standing height, cardiovascular and respiratory diseases,
mobility restriction, and reduced quality of life [2]. For this reason, it is
of significant importance for an early screening of sarcopenia to initiate
treatment soon.
The scientific literature is wide regarding the
influences of neuromuscular factors on the occurrence of sarcopenia and dynapenia. Some studies, for example, have shown that, at
the beginning of resistance exercise, gains related to muscle strength were not
due to intrinsic muscular physical capacity, but due to factors such as
increased activation and discharge of motor units [11,12]. Clark & Manini
showed that the decrease in load impacted a greater loss of muscle strength
than a decrease in lean mass [13]. In addition, neurological factors such as
excitation-contraction uncoupling in skeletal musculature and changes in
central command explained most of the loss of muscle strength [13].
Aging, in this sense, reduces the number of motor
units, in addition to influencing their reorganization, with the replacement of
type 2 motor units for type 1 motor units [14] and a decrease in the maximum
rate of action potential triggers of the motor units [12]. It also influences
the hyporeflexia of upper and lower motor neurons, as well as the decrease in
the conduction velocity of the nervous stimulus [15]. All these changes are
responsible for the muscular atrophy characteristic of the elderly [16]. For
this reason, sarcopenia is commonly associated with aging [8].
Thus, several factors that influence muscle strength
are emphasized, whether intrinsic to the muscle, such as the amount of lean
mass, or neuromuscular, associated with the activation capacity of the motor
plate, rate of triggering of action potentials, motor learning, and
contraction-excitatory synergy. However, there is little emphasis on the
influence of metabolic factors that can determine the decrease in muscle
strength.
Sarcopenic obesity and dynamic obesity: health consequences
Sarcopenic obesity is a condition that describes the
decline in muscle strength and lean mass in obese individuals, defined by the
coexistence of two diagnoses: obesity and sarcopenia [3,4,17]. The sum of the
two comorbidities leads to greater severity in the development of other
diseases than just one of them alone [3,4]. In addition, one condition can be a
precursor of the other. For example, obesity can be related to sedentary
behavior as it is a risk factor for the development of sarcopenia and
vice-versa [18]. The criteria for the coexistence of the diagnoses of obesity
and sarcopenia in sarcopenic obesity have been questioned [3,4,18] concerning
its efficiency, since the diagnostic criteria for sarcopenia used are aimed at
the elderly patient, as described by the EWGSOP2, for example.
Some studies in the literature used the term “dynamic
obesity” to refer to sarcopenic obesity, using the same concept [19,20,21]. Dynamic
obesity, however, refers to the initial idea of dynapenia
- the loss of muscle strength in obese patients - and perhaps it would be more
effective to define obesity as leading to decreased muscle strength [19]. While
sarcopenic obesity would be defined as obesity only with a decrease in lean
mass, without negative repercussions on strength or even with improvement, as
seen in studies [5,6].
A current limitation of studies on sarcopenic obesity
is the lack of diagnostic criteria that aims at the young population since
sarcopenic obesity is not restricted to the elderly [4], not properly isolating
neuromuscular factors from metabolic factors.
Physiological mechanisms of sarcopenic obesity
In obese individuals, there is an increase in the
deposit of lipids in the intramuscular environment due to the increase in
insulin resistance [22]. Sarcopenia, in this scenario, develops due to chronic
and systemic conditions of mild inflammation and increased body load [23].
Obesity is also a risk factor for systemic arterial
hypertension and hypercholesterolemia, conditions that can also influence
musculoskeletal function [1]. Hypertension can contribute to tissue damage
since it makes it difficult to exchange substrates necessary for its survival
[24], while hypercholesterolemia, in addition to being able to deregulate lipid
metabolism, which influences endothelial dysfunction, can be related to tissue
damage in tendons, the decrease in bone mineral density, and osteoarthritis
[25,26,27].
Muscle fibers are in a constant process of
degeneration and regeneration, resulting from the mechanism of inflammation and
tissue repair, respectively, being this behavior responsible for muscle growth
and remodeling [22]. Obesity also leads to metabolic complications that impair
angiogenesis and the formation of new muscle fibers [1]. This deregulation
leads to the deposition of fibrous tissue - from the tissue repair process -
and of adipose tissue, leading to structural and, consequently, functional loss
[28].
Obesity can also be related to a quantitative
imbalance in oxidative and glycolytic muscle fibers [29]. Oxidative muscle
fibers are endowed with a greater number of satellite cells, and as obesity
makes the activation of these cells difficult, the glycolytic muscle fibers
replace the functions of the oxidative fibers [1]. This change is an effect of
low-level chronic inflammation and negatively influences muscle regeneration
[1].
Obesity establishes an unfavorable environment for the
activation of satellite cells, preventing their proliferation and
differentiation into muscle fibers [30,31]. Macrophages attracted by the
chronic inflammatory process resulting from obesity can also inhibit satellite
cell activity [32]. Fibroadipogenic Progenitor Cells
or FAPs are cells that act in muscle repair, but in the absence of satellite
cell activity, they differentiate into fibroblasts and adipocytes,
characterizing the intramuscular lipid deposition [33] that sustains the
inflammatory condition of obesity. Figure 1 presents a proposal for a single
system associating muscular, neuromuscular, and metabolic factors.
Bariatric surgery: associations with sarcopenia and dynapenia
Bariatric surgery is the most used method and it has
shown the best results for the treatment of severe obesity in terms of weight
and body fat reduction, in addition to showing significant improvements in the
individual's morbidity and mortality [5,6,34,35,36]. Bariatric surgery sets the
patient to a phase of rapid weight loss after the intervention and a subsequent
phase of weight stability [6].
Due to the greater amount of total corporal mass, the
obese patient also has a greater amount of lean mass in comparison to a
non-obese individual [6]. During the phase of rapid weight loss, there is a
significant reduction in the amount of lean mass that can lead the individual
to develop sarcopenia [6].
Strategies for the prevention of sarcopenia and dynapenia
in bariatric surgery
Some studies showed that individuals who underwent
bariatric surgery showed improvements in muscle strength, even with a decrease
in lean mass [5,6]. When comparing women with sarcopenic obesity two years
after bariatric surgery to women with sarcopenic obesity who did not undergo
the same surgery [6], it was identified that the performance of the five
times-sit-to-stand-test was superior in the intervention group, even when both
groups were diagnosed with sarcopenic obesity. The result of this test for the
intervention group was still compatible with sarcopenia, according to the
EWGSOP2 criteria, but a significant improvement was demonstrated with the
compared group.
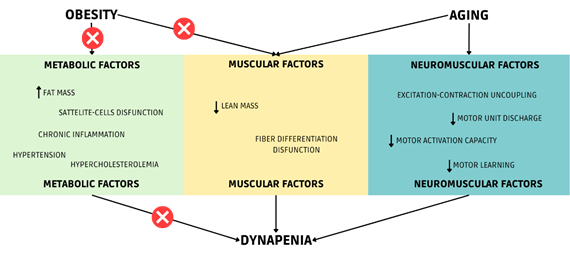
Source: Author himself
Figure 2 - Multifactorial system of dynapenia
in elderly obese patients after bariatric surgery
In the study of Coral et al. [5], individuals
were evaluated before bariatric surgery and compared six months after surgery.
Despite the significant reduction in lean mass, there were important
improvements in muscle performance, evaluated with gait speed and the “get up
and go test”.
Assuming that strength loss is linked to lean mass
loss is intuitive, but there are other factors, as seen in Figure 2, that can
more effectively impact – positively or negatively – muscle strength. In the
case of obesity, the rapid weight loss provided by surgery can reverse
metabolic factors that influence the occurrence of sarcopenia and dynapenia and may weigh even more than muscle factors and
neuromuscular factors.
Conclusion
The state of the art in sarcopenia demonstrates that
authors often assume that there is a causal relationship between sarcopenia and
dynapenia. The neglect of the use of the term dynapenia highlights this fact and restricts the
impressions about the existence of an intrinsic nature between the two terms.
For this reason, the better performance found in patients undergoing bariatric
surgery associated with higher muscle strength, despite the decrease in lean
mass, leads to a counterintuitive conclusion. There are several factors -
muscular, neuromuscular, and metabolic - for muscular performance, in addition
to different weights to be considered for each of these influences that can
unbalance negatively or positively. Thus, further studies are needed to
investigate the nature of the sarcopenia-dynapenia
relationship and the weight of the influence of other factors on these
conditions.
Potential conflict of interest
No conflicts of interests have been reported for this
article.
Funding source
The present study was carried out without funding.
Authors’ contributions
Research conception and design: Takenami LI, Santos CPC, Bomfim ES, Filha AMSG, Literature
review: Takenami LI, Lagares LS, Almeida LAB; Manuscript writing: Takenami LI, Lagares LS, Macedo
RC; Critical review of the manuscript for important intellectual content: Santos
CPC, Filha AMSG, Bomfim ES,
Almeida LAB
References
- Collins KH, Herzog W, MacDonald GZ, Reimer RA, Rios JL, Smith IC, et al.
Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory
pathways suggest a central role for loss of muscle integrity. Front
Physiol 2018;9. doi: 0.3389/fphys.2018.00112 [Crossref]
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age and Ageing 2019;48:16-31. doi: 10.1093/ageing/afy169 [Crossref]
- Ciudin A, Simó-Servat A, Palmas F, Barahona MJ. Sarcopenic obesity: a new challenge in the clinical practice. Endocrinol Diabetes Nutr 2020;10:672-81. doi: 10.1016/j.endinu.2020.03.004 [Crossref]
- Barazzoni R, Bischoff S, Boirie Y, Busetto L, Cederholm T, Dicker D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr 2018 (6 Pt A):1787-93. doi: 10.1016/j.clnu.2018.04.018 [Crossref]
- Coral RV, Bigolin AV, Machry MC, Menguer RK, Pereira-Lima JC, Contin
I, et al. Improvement in muscle strength and metabolic
parameters despite muscle mass loss in the initial six months after bariatric
surgery. Obes Surg
2021;31(10):4485-91. doi: 10.1007/s11695-021-05634-0 [Crossref]
- Buzza AFB, Machado CA, Pontes F, Sampaio LG,
Contador JS, Sampaio CL, et al. Prevalence of sarcopenia in women at
stable weight phase after Roux-en-Y gastric bypass. Arch Endocrinol Metab 2022:2359-3997000000494. doi: 10.20945/2359-3997000000494 [Crossref]
- Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care 2010;13(3):271-6. doi: 10.1097/MCO.0b013e328337819e [Crossref]
- Dynapenia S, Clark BC,
Manini TM. Sarcopenia 6 ¼ Dynapenia. J Gerontol [Internet] 2008 [cited 2022 Jan 28];63(8):829-34.
Available from:
http://www.insideoutsidespa.com/archive/Clark_Sarcopenia_Dynapenia.pdf
- Delmonico MJ, Beck DT. The current understanding of sarcopenia: Emerging tools and interventional possibilities. Am J Lifestyle Med 2017;11:167-81. doi: 10.1177/1559827615594343 [Crossref]
- Chao YP, Fang WH, Chen WL, Peng TC, Yang WS, Kao TW. Exploring muscle
health deterioration and its determinants among community-dwelling older
adults. Front Nutr 2022;9. doi: 10.3389/fnut.2022.817044 [Crossref]
- Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: Mechanisms and recommendations for training practices. Sport Med 2006;36(2):133-49. doi: 10.2165/00007256-200636020-00004 [Crossref]
- Kamen G. Aging, resistance training, and motor unit discharge behavior. Can J Appl Physiol 2005;30(3):341-51. doi: 10.1139/h05-126 [Crossref]
- Clark BC, Manini TM, Bolanowski SJ, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting: II. Neurological properties and motor imagery efficacy. J Appl Physiol 2006;101(1):264-72. doi: 10.1152/japplphysiol.01402.2005 [Crossref]
- Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr 1997;127:1011-3. doi: 10.1093/jn/127.5.1011S [Crossref]
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 2004;82(4):238-48. doi: 10.1139/y04-017 [Crossref]
- Delbono O. Neural control of aging skeletal muscle. Aging Cell 2003;2(1):21-9. doi: 10.1046/j.1474-9728.2003.00011.x [Crossref]
- Kwon YN, Yoon SS, Lee KH. Sarcopenic obesity in elderly Korean women: A nationwide cross-sectional study. J Bone Metab 2018;25(1):53-8. doi: 10.11005/jbm.2018.25.1.53 [Crossref]
- Donini LM, Busetto
L, Bauer JM, Bischoff S, Boirie Y, Cederholm T, et
al. Critical appraisal of definitions and diagnostic criteria for sarcopenic
obesity based on a systematic review. Clin Nutr 2020
39(8):2368-88. doi: 10.1016/j.clnu.2019.11.024 [Crossref]
- Scott D, Chandrasekara SD, Laslett LL, Cicuttini F, Ebeling PR, Jones G. Associations of sarcopenic obesity and dynapenic obesity with bone mineral density and incident fractures over 5-10 years in community-dwelling older adults. Calcif Tissue Int 2016;99(1):30-42. doi: 10.1007/s00223-016-0123-9 [Crossref]
- Rossi AP, Bianchi L, Volpato S, Bandinelli S, Guralnik J, Zamboni M, et al. Dynapenic abdominal obesity as a predictor of worsening disability, hospitalization, and mortality in older adults: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2017;72(8):1098-104. doi: 10.1093/gerona/glw203 [Crossref]
- Batsis JA, Zbehlik AJ, Pidgeon D, Bartels SJ. Dynapenic obesity and the effect on long-term physical function and quality of life: Data from the osteoarthritis initiative physical functioning, physical health and activity. BMC Geriatr 2015;15(1). doi: 10.1186/s12877-015-0118-9 [Crossref]
- Akhmedov D, Berdeaux R. The effects of obesity on skeletal muscle
regeneration. Front Physiol 2013;4:1-12. doi: 10.3389/fphys.2013.00371 [Crossref]
- Hoy D, Geere JA, Davatchi
F, Meggitt B, Barrero LH. A time for action:
Opportunities for preventing the growing burden and disability from
musculoskeletal conditions in low- and middleincome
countries. Best Pract Res Clin Rheumatol
2014;28(3):377-93. doi: 10.1016/j.berh.2014.07.006 [Crossref]
- McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 2015;116(6):1022-33. doi: 10.1161/CIRCRESAHA.116.303697 [Crossref]
- Tilley BJ, Cook JL, Docking SI, Gaida JE. Is higher serum cholesterol associated with altered tendon structure or tendon pain? A systematic review. Br J Sports Med. 2015;49(23):1504-9. doi: 10.1136/bjsports-2015-095100 [Crossref]
- Farnaghi S, Prasadam I, Cai G, Friis T, Du Z, Crawford R, et al. Protective effects of mitochondria-targeted antioxidants and statins on cholesterolinduced osteoarthritis. FASEB J 2017;31(1):356-67. doi: 10.1096/fj.201600600R [Crossref]
- Makovey J, Chen JS,
Hayward C, Williams FMK, Sambrook PN. Association between serum cholesterol and
bone mineral density. Bone 2009;44(2):208-13. doi: 10.1016/j.bone.2008.09.020 [Crossref]
- Karalaki M, Fili S, Philippou A, Koutsilieris M.
Muscle regeneration: Cellular and molecular events. In Vivo (Brooklyn)
[Internet] 2009 [cited 2022 Jan 21];23(5):779-96. Available from:
https://pubmed.ncbi.nlm.nih.gov/19779115/
- Pattanakuhar S, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. The effect of exercise on skeletal muscle fibre type distribution in obesity: From cellular levels to clinical application. Obes Res Clin Pract 2017;11(5):112-32. doi: 10.1016/j.orcp.2016.09.012 [Crossref]
- D’Souza DM, Trajcevski KE, Al-Sajee D, Wang DC, Thomas M, Anderson JE, et al. Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. Physiol Rep 2015;3(8):1-12. doi: 10.14814/phy2.12506 [Crossref]
- Meng J, Bencze M, Asfahani R, Muntoni F, Morgan JE. The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet Muscle 2015;5(1):1-12. doi: 10.1186/s13395-015-0036-8 [Crossref]
- Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 2010;298(5). doi: 10.1152/ajpregu.00735.2009 [Crossref]
- Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 2011;1(1):1-20. doi: 10.1186/2044-5040-1-21 [Crossref]
- Pauleau G, Goin G, Goudard Y, De La Villeon B, Brardjanian S, Balandraud P. Influence of age on sleeve gastrectomy results. J Laparoendosc Adv Surg Tech 2018;28(7):827-32. doi: 10.1089/lap.2017.0696 [Crossref]
- Gil S, Kirwan JP, Murai IH, Dantas WS, Merege-Filho CAA,
Ghosh S, et al. A randomized clinical trial on the effects of exercise on
muscle remodelling following bariatric surgery. J
Cachexia Sarcopenia Muscle 2021;12(6):1440-55. doi: 10.1002/jcsm.12815 [Crossref]
- Voican CS, Lebrun A, Maitre S, Lainas P, Lamouri K, Njike-Nakseu M, et al. Predictive score of sarcopenia occurrence one year after bariatric surgery in severely obese patients. PLoS One 2018;13(5):1-12. doi: 10.1371/journal.pone.0197248 [Crossref]
