Rev Bras Fisiol Exerc. 2022;21(6):329-39
doi: 10.33233/rbfex.v21i6.5409
ORIGINAL ARTICLE
Glucose threshold: concurrent validity with lactate
threshold and concordance with heart rate variability threshold
Limiar
glicêmico: validade com limiar de lactato e concordância com a variabilidade da
frequência cardíaca
Luciana Carletti, Igor Ziviani Araujo, Leticia Nascimento dos Santos Neves, Victor Hugo
Gasparini Neto, Richard Diego Leite
Universidade
Federal do Espírito Santo, Vitoria, ES, Brazil
Received: September 10, 2022; Accepted: November 23,
2022.
Correspondência:
Luciana Carletti, E-mail: luciana.carletti@ufes.br
How to cite
Carletti L, Araujo IZ, Neves LNS, Gasparini Neto VH, Leite RD. Glucose
threshold: concurrent validity with lactate threshold and concordance with
heart rate variability threshold. Rev Bras Fisiol Exerc. 21(6):329-39. doi:
10.33233/rbfex.v21i6.5409
Abstract
Introduction: At lactate
threshold (LT2) intensity, there is an increase in the activity of the
sympathetic nervous system, an increase in plasma catecholamines, and an
increase in blood glucose, which represent the glucose threshold (GT) and the
heart rate variability threshold (HRVT). These thresholds may concord and allow
exercise prescription through more accessible means. Aim: To analyze the
concurrent validity of GT with LT2 and assess whether there is a concordance
between GT and HRVT. Methods: 31 healthy and active men (22 ± 2 years)
underwent two days of cardiopulmonary exercise testing (CPET). On the second
day, the test aimed to identify GT, LT2, and HRVT. The intraclass correlation
coefficient (ICC), typical error (TE), coefficient of variation (CV), and
Bland-Altman tested their reliability and concordance. Results: The HR showed
good reliability (ICC = 0.80) and good precision (TE = 4.7% and CV = 6.6%) for
GT with LT2. For GT and HRVT, the HR showed moderate reliability (ICC = 0.60)
and good precision (TE = 5.9% and CV = 8.4%). Conclusion: GT and LT2
presented concurrent validity for identifying the second anaerobic threshold.
GT concordance with LT2 is reinforced by the mechanisms that link them.
Keywords: anaerobic threshold; exercise
intensity; blood glucose; heart rate.
Resumo
Introdução: Na intensidade do limiar de lactato
(LL2) ocorre um aumento da atividade do sistema nervoso simpático, aumento das
catecolaminas plasmáticas e da glicemia, que representam o limiar glicêmico
(LG) e o segundo limiar de variabilidade da frequência cardíaca (LVFC2). Esses
limiares podem apresentar concordância e permitir a prescrição do exercício por
meios mais acessíveis. Objetivo: O objetivo do estudo foi analisar a
validade concorrente do limiar glicêmico com o limiar de lactato; e sua
concordância como o limiar de variabilidade da frequência cardíaca para
identificação do segundo limiar anaeróbio. Métodos: 31 homens saudáveis
e ativos (22 ± 2 anos) foram submetidos a um protocolo escalonado de Teste
Cardiopulmonar de Exercício (TCPE), monitorados por medidas de glicemia, lactacemia e variabilidade da frequência cardíaca para a
identificação do LG, LL2 e LVFC2. O coeficiente de correlação intraclasse (CCI), erro típico (ET), coeficiente de
variação (CV) e Bland-Altman testaram a
confiabilidade e concordância. Resultados: A HR apresentou boa
confiabilidade (ICC = 0,80) e boa exatidão (ET = 4,7% e CV = 6,6%) para o LG
com LL2. Para o LG e LVFC2, a FC apresentou confiabilidade moderada (ICC =
0,60) e boa precisão (ET = 5,9% e CV = 8,4%). Conclusão: O LG e o LL2
apresentaram validade concorrente para identificação do segundo limiar
anaeróbio. A concordância entre o LG e o LVFC2 é reforçada pelos eventos
fisiológicos que os relacionam.
Palavras-chave: limiar anaeróbio; intensidade do
exercício; glicemia; frequência cardíaca.
Introduction
The definition of more precise
intensities for the practice of physical exercises identifying the metabolic
thresholds favors the determination of individualized training zones and more
efficient improvements in the parameters of physical fitness related to health
[1] or performance [2]. Metabolic thresholds consist of submaximal
physiological index measured during an incremental exercise test.
The anaerobic threshold (AT) is
widely used in the science of sports [3,4] being a physiological phenomenon
that can be identified using different parameters, for example, ventilatory
threshold (LV), lactate threshold (LT), heart rate variability threshold
(HRVT1 and 2), glucose threshold (GT), among others [3,5]. Traditionally, LT and VT
have been used for assessing this phenomenon [6,7]. The LT can be identified at
two distinct moments: aerobic and anaerobic threshold (Lactate Threshold 2 –
LT2) [8]. It is known that LT2 is characterized by the exponential increase of
lactate in the blood and that, when considered fixed values, it is
approximately 4 mmol·L-1 [8].
This phenomenon occurs at exercise
intensities with increased plasma catecholamines [9]. The plasma catecholamine
increase promotes the bioavailability of blood glucose through glycogenolysis
and gluconeogenesis stimulation [10]. The scientific literature indicates a
concordance between the glycemic and second ventilatory methods for anaerobic
threshold determination in patients with type 2 diabetes [11].
A growing body of evidence
indicated the AT determination by glucose levels, called Individual Glucose
Threshold (IGT) [12]. The authors observed that blood glucose levels reduced
while blood lactate increased (LT) [12]. The lowest glucose value defined the
IGT before the abrupt increase associated with LT2 in runners who performed a
track test [12].
In addition, a physiological
relationship is expected between glycemic response during exercise and heart
rate variability due to the sympathetic nervous system of the effect on glucose
metabolism [10]. The literature suggests using HRV to estimate physiological
thresholds, such as the ventilatory or lactate threshold, in an incremental
test [13]. It is believed that HRVT can identify the intensity of effort
similar to that of GT because, in exercises with higher intensities, the
sympathetic nervous system is predominant [14], and factors that prove the
increase in blood glucose are also observed [10].
Therefore, it is necessary to
analyze the concurrent validity of the glucose threshold with the lactate
threshold and their concordance on the heart rate variability threshold to
identify the anaerobic threshold in a more accessible way. We hypothesized that
GT would have a good concordance with LT2 and HRVT.
Methods
Sample
The sample was composed of 31
university students, healthy, male, age 22 ± 2 years old. The participants were
informed about the procedures. Inclusion criteria: to perform a physical
exercise (evaluated by the usual level of physical activity ≥ 150
min·week-1) and between 18 and 30 years old. Exclusion criteria
were: medication use, locomotor limitations or diagnosed diseases, smoking
story, and alcohol. The research ethics committee approved the study of the Universidade Federal do Espírito
Santo. (CAAE 76607717.5.0000.5542).
Procedures
The procedures were performed as
shown in Figure 1. The health and personal information survey was done through a self-completed questionnaire. First, the
participants were eligible, respecting the inclusion and exclusion criteria.
Subsequently, there was a visit to the laboratory in two days, with an interval
of 48 hours. On the first day, anamnesis, anthropometric assessment, and cardiopulmonary
test were performed with blood collection from the earlobe, rest, and recovery.
In addition, cardiopulmonary testing was applied for medical screening and
familiarization. On the second day, the second cardiopulmonary test (step
protocol) was performed due to the stability necessary for a good collection of
heart rate variability and blood collection at rest, during the test, and
during recovery. Finally, glucose, lactate, and heart rate variability
thresholds were identified. Three participants were excluded due to the
difficulty in identifying the glycemic threshold, as described in the section
on glycemic threshold collection and identification. (Figure 1).
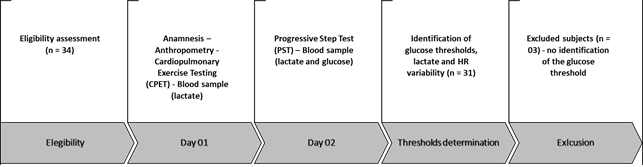
Figure 1 – Study design
Anthropometry
Body mass and height were measured
using a digital anthropometric scale with a 1mm precision stadiometer (Marte Cientifica, L200, São
Paulo), with a maximum capacity of 201 kg and a sensitivity of 50 g to
calculate the body mass index (BMI). The body composition was measured using a
scientific skinfolds caliper with 0.1 mm sensitivity and 85mm reading amplitude
(Mitutoyo/Cescorf, RS), including seven skinfolds
(tricipital, subscapular, pectoral, mid-axillary, suprailiac,
abdominal and thigh). Finally, the fat percentage was calculated using the
formula proposed by Jackson and Pollock [15].
Cardiopulmonary Exercise Test – (CPET)
The individuals underwent
cardiological assessment of rest and exertion to assess their health conditions
and define ventilatory threshold 1 (VT1) intensity. The velocity corresponding
to VT1 was the parameter adopted to determine the start speed of the
progressive step test.
Ventilatory variables were measured
using the metabolic gas analyzer Cortex Metalyzer 3b
(Leipzig, Germany) with breath-by-breath collection and analyzed using the MetasoftTM software. The equipment was calibrated using a
known gas mixture (11.97% O2 and 4.95% CO2 original,
certified by the manufacturer). The system was calibrated again at each new
test using the ambient air reference. The volume sensor was calibrated using a
3L syringe (Hans Rudolph, Oklahoma, USA). Collecting masks of varying sizes
were used according to the facial dimensions of each subject. The test was
monitored by a cardiologist and a physical education professional. There was
verbal encouragement in the final phase of the CPET, aiming at reaching maximum
effort. At least three of the following criteria were used to identify the test
as the maximum: a) voluntary exhaustion reported immediately after the test; b)
heart rate of at least 90% of predicted for the age (220-age); c) respiratory
exchange ratio (RER) equal to or above 1.05; d) maximum oxygen consumption,
observed by the plateau or peak concept [16].
Progressive Step Test (PST)
At least 48 hours after the first
visit, the participants performed a new exertion test. The protocol used was a
continuous load increment, 3 min per stage, with an increment of 1 km·h-1
every 3 min. Before the test, the participant remained at rest, being 5min
supine and 5min standing. The protocol's initial speed was 4 km·h-1
below the VT1 identified by the first CPET and continued up to the maximum
effort, not exceeding 10 exercise stages.
Glucose Threshold (GT) and Lactate Threshold (LT)
During the rest period, 25 µL of
arterialized blood were collected from the earlobe, without hyperemia, to
determine the blood glucose and blood lactate concentration. Blood was
immediately transferred to 1.5 ml capped polyethylene microtubules containing 5
µL sodium fluoride (1%) and packaged for further analysis.
During exercise, a blood sample was
collected from the earlobe (25 µL) between each stage. At the end of each
stage, the participant jumped off the treadmill, and soon after collection, he
returned. They were later analyzed for blood glucose, and blood lactate values
(mmol·L-1) were collected [6]. The YSI 2300 STAT Plus Glucose and
Lactate Analyzer (Ohio, USA) was used. For joint analysis, blood samples were
stored under refrigeration (-80°C).
The criterion to determine at which
stage the glucose threshold occurred was the lowest glucose value before an
abrupt increase [17]. Lactate Threshold 2 was identified by the second
linearity breakpoint and exponential lactate accumulation [18,19].
Heart Rate Variability Threshold
A Polar H7 Heart Sensor connected
via Bluetooth with a smartphone was used to collect the heart rate variability
(HRV) and beat-to-beat (R-R intervals). Each stage described in the step test
was recorded in the Elite HRV app (Elite HRV, Asheville-NC) [20] and edited in
the Kubios HRV Standard 3.0 software [21]. Data at
rest and immediately after the end of the exercise test were used for analysis.
R-R intervals were grouped into
three-minute sequences for analysis of HRV. Data filtering was performed in the
Kubios software when there were demonstrations of
interference in the data. The first 90 seconds of physical activity at each
stage were excluded from the analysis due to the adjustment time for HR and HRV.
This study chose the Root Mean
Square of the Successive Differences (RMSSD) and Poincaré
plot indexes (SD1 and SD2) as the HRV indexes (time-domain and non-linear HRV
parameters). To analyze the HRVT, the variables RMSSD and SD1 were used,
defined as RMSSD – analysis of heart rate variability in the time domain,
calculated by the root mean square of the successive differences between
adjacent R-R intervals and SD1 – analysis of heart rate variability by the
geometric method (Poincaré plot). It represents the
short-term analysis of the variability of the R-R interval.
The HRVT was determined by the
linearity breakpoint after the lowest value, with a subsequent increase in the
RMSSD and SD1 confirmed by the linearity break of the SD1/SD2 variables only
when necessary (determined by visual inspection) [22,23]. They were identified
in Excel software (Microsoft Excel® 2022).
Statistical procedures
All data were tabulated and
double-verified by independent researchers. The normality was tested using the
Shapiro-Wilk test and submitted to evaluate the histogram, kurtosis, and
skewness. The results were presented as mean ± standard deviation (SD). To
compare the identification methods of GT with LT2 and of GT with HRVT, the
paired Student’s “t” test was used. Reproducibility, reliability, and precision
were comprehended by the intraclass correlation coefficient (ICC), typical
error (TE), and coefficient of variation (CV) tests. For ICC, values < 0.5
indicate low reliability, 0.5 – 0.75 moderate, 0.75 – 0.90 good, and > 0.90
excellent reliability [24]. To present good reproducibility and reliability,
the TE and CV values must be below the cutoff point, 10% and 20% [25],
respectively. The Bland Altman technique was used to analyze the concordance between
the methods. The analysis was performed using SPSS 20.0 software, and the
figures were created by GraphPad Prism 6. The significance level adopted was P
< 0.05.
Results
Table I shows the characteristics
of the subjects, who present normal BMI and fat percentage according to the WHO
[15]. The performance variables confirm that the maximum effort was achieved in
the Cardiopulmonary Exercise Test.
Table I - Anthropometric and maximal
physiological characteristics of participants at Cardiopulmonary Exercise Test.
(n = 31)

Values of mean ± SD. BMI = Body
Mass Index; [La] = Lactate concentration; Max = maximal; HRmax
= Maximal Heart rate; VO2max = Maximal oxygen consumption; RER =
Respiratory exchange ratio; RR = Resting R-R interval; SD = Standard deviation
No differences were found (Table
II) between the GT vs. LT2 and GT vs. HRVT methods for variables: speed, VO2,
%VO2, HR, %HR, lactate, and RER (P > 0.05).
Table II - Comparison between
identification methods
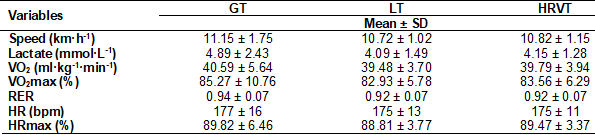
Paired Student’s “t” test, GT vs.
LT2 and GT vs. HRVT. No significant difference (P>0.05). Values of Mean ±
SD, Standard deviation (SD). Data were compared using paired t-test. Glucose
threshold (GT), Heart rate (HR), Heart rate variability threshold (HRVT2),
Lactate Threshold (LT), Relative and absolute oxygen consumption (VO2)
and Respiratory exchange ratio (RER)
In Table III, the GT accuracy was
tested using the LT2 as a reference. The GT shows moderate reliability (ICC)
for VO2 (0.55) and good reliability for HR (0.80). In addition,
there was good reproducibility for VO2: TE = 3.8 (9.4%) and CV =
13.3%; and for HR: TE = 8.2 (4.7%) and CV = 6.6.
GT and HRVT were also compared and
exhibited values of TE = 10.4 (5.9%), CV = 8.4%, and ICC = 0.60, only for the
HR variable, which showed moderate reliability, good reproducibility, and
precision (Table III). Speed (CV = 18.0) and VO2 (CV = 16.3)
presented values within the cutoff point. However, a greater variety of data
was interpreted with moderate concordance (Table III).
Table III – Values of
Typical Error (TE), Coefficient of variation (CV), and Intraclass Correlation
Coefficient (ICC) between the methods of GT vs. LT2 and GT vs. HRVT
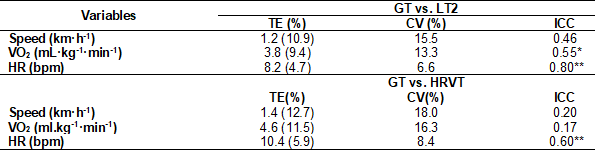
Glucose Threshold (GT), Heart rate
(HR), and Heart rate variability threshold (HRVT), Lactate Threshold (LT),
Relative and absolute oxygen consumption (VO2), *P < 0.05; and
**P < 0.01 for ICC
Figure 2 shows the concordance
values between LT2 and GT (A, C, and E) and GT and HRVT (B, D, and F). There
was good concordance in the variables, VO2, HR, and speed in the
comparison between LT2 and GT and GT and HRVT, which presented a mean of the
differences close to zero.
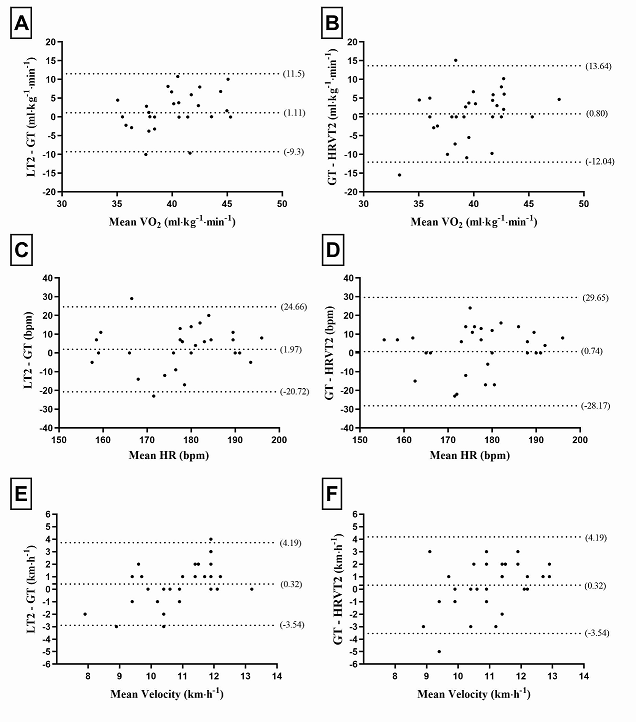
Bland-Altman plot: Y-axis – upper tween
means), and lower dashed line (indicates lower limit – 2SD). Glucose Threshold
(GT), Heart rate (HR), and Heart rate variability threshold (HRVT), Lactate
Threshold (LT2), Relative and absolute oxygen consumption (VO2).
Figure 2 – Limits of concordance of the
Bland Altman technique between the methods of GT vs. LT2 and GT vs. HRVT
Discussion
The present study confirms the
hypothesis that there is good concordance between GT and LT2, specifically for
the HR and VO2 parameters. This finding stands out because it
enables the use of GT interchangeably with LT2 to identify the anaerobic threshold.
The lactate threshold is one of the
most used methods to identify physiological thresholds. However, there is a
demand for expensive equipment (laboratory lactate analyzer) to identify it. It
is also possible to use portable equipment; however, the cost is higher than a
portable glucometer. On the other hand, the GT method is considered a more
straightforward test with a lower price and is accessible [26]. However, in
this present study, the blood glucose analysis was carried out using expensive
equipment. Despite this, other investigations employed measures with portable
equipment showing reliable [11,27].
The running speed corresponding to
the GT seems to be a good predictor of LT2 and maximal lactate steady state
(MLSS) for inactive men who performed the treadmill test [26] and tests
performed on track with subjects physically active [28]. In addition, our
findings showed statistical similarities between glucose, VO2, and
HR variables. Emphasizing mainly VO2 and HR, which are essential parameters
for exercise prescription [2].
Therefore, the present study
presents the finding that the phenomenon is also observable during a CPET,
which consists of a stress assessment, is more applied in clinical settings,
and can be used in populations with comorbid conditions [11]. In this sense,
these findings make the GT method more accessible. This test can be included as
a complementary measure in clinical trials when there is interest in accessing
more individualized information for exercise prescriptions. For example, one
study analyzed the response in cardiorespiratory capacity in sedentary men and
women, comparing an individualized training program with pre-established
training zones based on percentages of HRmax. The
same concluded that individualized training had a more significant effect on
the response of cardiorespiratory capacity [29]. Therefore, using thresholds to
determine training zones can be an excellent option to obtain more efficient
results.
In this study, GT and LT2 were
identified at an intensity of 89.8% and 88.8% of FCmax,
respectively, demarcating very close intensities when one or the other method
was used. This finding helps to reinforce the similarity between the two
methods corresponding to AT. This protocol makes it possible to use the GT to
identify the AT for professional use in practice.
Moreover, if you collect HR values
during the GT test, it will be possible to use them to control exercise
intensities. For example, studies that performed the prescription
individualized by threshold with non-athlete adults observed significant
improvements in ventilatory efficiency, tolerance to maximum and submaximal
exercise [1], and cardiorespiratory fitness [29].
Another aim of this study was to
test whether the GT concords with the HRVT to clarify if their relationship is
observed in identifying the AT. Some physiological events may explain the
proximity of GT and LT2 since there is a relationship between the increase in
plasma glucose and the accumulation of lactate. At exercise intensities
corresponding to the AT, plasma catecholamine concentration is high. These
biomarkers act concomitantly as neurotransmitters and hormones with
hyperglycemic action; they break down hepatic glycogen (glycogenolysis) and stimulate
gluconeogenesis from lactate, alanine, and glycerol, increasing blood glucose
levels [10,30]. In addition, high levels of catecholamines inhibit insulin
release, contributing to the hyperglycemic effect [10].
In this context, the blood lactate
increases induce a stimulus for the feedback of peripheral metaboreceptors to
stimulate the action of the cardiovascular control center, further increasing
the stimulation of the sympathetic nervous system, which affects additional
increases in cardiac activity. Thus, it is possible to observe that the
concordance observed in the present study between the HRVT, which is an
indicator of cardiac autonomic balance, and the GT has physiological
explanations and reinforces the use of the GT for the prescription of exercise
intensity.
Conclusion
In conclusion, our results suggest
that GT is a valid method to identify the anaerobic threshold, having LT2 as a
reference. Furthermore, the concordance between the GT and the HRVT reinforces
the method’s reliability, as their mechanisms are related. Therefore, GT
employing the FC for prescription offers an option to replace the traditional
LT2 method in physically active young adults.
Academic affiliation
This article is the result of the
Institutional Scientific Initiation Program of the Federal University of Espírito Santo (UFES), by class student Igor Ziviani Araújo, supervised by Professor Luciana Carletti, UFES.
Conflict of interest
All authors are responsible for the
manuscript's content and approve its final version. No commercial party that
supports this article and has a direct financial interest in the research
results confers or will confer financial benefits on the authors or any
organization with which the authors are associated. The authors declare that no
known competing financial conflicts of interest or personal relationships may
have influenced the work reported in this article.
Funding
The present work was carried out
with the support of a scientific initiation scholarship from the Universidade Federal do Espírito
Santo.
Authors’ contribution
Conception and design of the
research: Carletti L, Araujo IZ, Neves LNS, Gasparini
Neto VH, Leite RD; Data
collection: Araujo IZ, Neves LNS, Gasparini Neto VH;
Data analysis and interpretation: Carletti L, Araujo
IZ, Neves LNS, Gasparini Neto VH, Leite
RD; Statistical analysis: Carletti L, Araujo IZ,
Neves LNS; Writing of the manuscript: Carletti L,
Araujo IZ, Neves LNS, Gasparini Neto VH, Leite RD; Critical review of the manuscript for important
intellectual content: Carletti L, Araujo IZ, Neves
LNS, Gasparini Neto VH, Leite
RD
References
- Peric R, Drobnic FM, Baker JS. Feasibility of individualized aerobic
threshold-based exercise on ventilatory efficiency in sedentary adult asthma
patients. Minerva Pneumol. 2018;57(4):92-8. doi: 10.23736/S0026-4954.18.01829-1 [Crossref]
- Weatherwax RM, Harris NK, Kilding
AE, Dalleck LC. The incidence of training
responsiveness to cardiorespiratory fitness and cardiometabolic measurements
following individualized and standardized exercise prescription: study protocol
for a randomized controlled trial. Trials. [Internet]. 2016 [citado 2022 Jan
8];17(1):601. Disponível em:
http://trialsjournal.biomedcentral.com/articles/10.1186/s13063-016-1735-0
- Azevedo
PHSM, Garcia A, Duarte JMP, Rissato GM, Carrara VKP, Marson RA. Limiar Anaeróbio e Bioenergética: uma abordagem
didática. Revista da Educação Física/UEM 2009 [citado 2022 Jan 8];20(3):453-64. doi: 10.4025/reveducfis.v20i3.4743 [Crossref]
- Poole DC, Rossiter HB, Brooks GA, Gladden LB. The
anaerobic threshold: 50+ years of controversy. J Physiol. 2021;599(3):737–67. doi: 10.1113/JP279963
- Peinado AB, Rojo JJ, Calderón FJ, Maffulli N. Responses to increasing exercise upon reaching the anaerobic threshold, and their control by the central nervous system. BMC Sports Sci Med Rehabil. 2014;6(1):1-7. doi: 10.1186/2052-1847-6-17 [Crossref]
- Gaskill SE, Ruby BC, Walker AVAJ, Sanchez OA, Serfass RC, Leon AS. Validity and reliability of combining three methods to determine ventilatory threshold. Med Sci Sport Exerc. 2001;33(11):1841-8. doi: 10.1097/00005768-200111000-00007 [Crossref]
- Okano AH, Altimari LR, Simões HG, Moraes AC de, Nakamura
FY, Cyrino ES, et al. Comparação entre limiar anaeróbio determinado por variáveis
ventilatórias e pela resposta do lactato sanguíneo em ciclistas. Rev
Bras Med do Esporte. 2006;12(1):39-44. doi: 10.1590/S1517-86922006000100008 [Crossref]
- Kindermann W, Simon G, Keul J. The significance of the aerobic-anaerobic transition for the determination of work load intensities during endurance training. Eur J Appl Physiol Occup Physiol. 1979;42(1):25-34. doi: 10.1007/BF00421101 [Crossref]
- Chmura J, Nazar K, Kaciuba-Uscilko H. Choice reaction time during graded exercise in relation to blood lactate and plasma catecholamine thresholds. Int J Sports Med. 1994;15(4):172-6. doi: 10.1055/s-2007-1021042 [Crossref]
- Powers SK, Howley ET. Fisiologia do Exercício: Teoria e aplicação ao
condicionamento e ao desempenho. 9 ed. São Paulo: Manole; 2017. 672 p.
- Delevatti RS, Kanitz AC, Alberton CL, Marson EC, Pantoja PD, Pinho CDF, et al. Glycemic threshold as an alternative method to identify the anaerobic threshold in patients with type 2 diabetes. Front Physiol. 2018;9:1-8. doi: 10.3389/fphys.2018.01609 [Crossref]
- Simões HG, Silvia C, Campbell G, Kokubun E. Determinação do limiar anaeróbio por meio de dosagens glicêmicas e lactacidêmicas em testes de pista para corredores. Rev Paul Educ Fís. 1998;12(1):17-30. doi: 10.11606/issn.2594-5904.rpef.1998.139529 [Crossref]
- Karapetian GK, Engels HJ, Gretebeck RJ. Use of heart rate variability to estimate LT and VT. Int J Sports Med. 2008;29:652-7. doi: 10.1055/s-2007-989423 [Crossref]
- Perrout JR, Dal’Molin MAP. Heart rate variability threshold. Med Sci
Sport Exerc. [Internet].
1998 [citado 2022 Jan12];30(Suppl):250. Disponível
em: http://journals.lww.com/00005768-199805001-01423
- Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497-504. doi: 10.1079/BJN19780152 [Crossref]
- Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27 p. 1292-301. doi: 10.1249/00005768-199509000-00009 [Crossref]
- Simões HG, Grubert Campbell CS, Kokubun E, Denadai BS, Baldissera V. Blood glucose responses in humans mirror lactate responses for individual anaerobic threshold and for lactate minimum in track tests. Eur J Appl Physiol Occup Physiol. 1999;80(1):34-40. doi: 10.1007/s004210050555 [Crossref]
- Kindermann W, Simon G, Keul J. The significance of the aerobic-anaerobic transition for the determination of work load intensities during endurance training. Eur J Appl Physiol Occup Physiol. 1979 Sep;42(1):25-34. doi: 10.1007/BF00421101 [Crossref]
- Pires
FO, Silva AEL, Gagliardi JFL, Barros RV, Kiss MAPDM. Caracterização da curva do
lactato sanguíneo e aplicabilidade do modelo Dmax
durante protocolo progressivo em esteira rolante. Rev
Bras Med do Esporte
[Internet]. 2006 [citado Fev 2022];12(2):71-5.
Disponível em:
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1517-86922006000200003&lng=pt&tlng=pt
- Perrotta AS, Jeklin AT, Hives BA, Meanwell LE, Warburton DER. Validity of the elite HRV smartphone application for examining heart rate variability in a field-based setting. J Strength Cond Res [Internet]. 2017 Aug;31(8):2296-302. doi: 10.1519/JSC.0000000000001841 [Crossref]
- Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-aho PO, Karjalainen PA. Kubios HRV – Heart rate variability analysis software. Comput Methods Programs Biomed [Internet]. 2014 [citado 2022 Fev
12];113(1):210-20. Disponível em: https://linkinghub.elsevier.com/retrieve/pii/S0169260713002599
- Mankowski RT, Michael S, Rozenberg R, Stokla S, Stam HJ, Praet SFE. Heart-rate variability threshold as an alternative for spiro-ergometry testing. J Strength Cond Res. 2017;31(2):474-9. doi: 10.1519/JSC.0000000000001502 [Crossref]
- Nascimento EMF, Kiss MAPDM, Santos TM, Lambert M, Pires FO. Determination of lactate thresholds in maximal running test by heart rate variability data set. Asian J Sports Med [Internet]. 2017 Aug 9. doi: 10.5812/asjsm.58480 [Crossref]
- Koo TK, Li MY. A Guideline of selecting and reporting
intraclass correlation coefficients for reliability research. J Chiropr Med [Internet]. 2016;15(2):155-63. doi: 10.5812/asjsm.58480 [Crossref]
- Hopkins WG. Correlation coefficient: a new view of
statistics [Internet]. 2000
[citado 2020 Fev 17]. Disponível em:
http://www.sportsci.org/resource/stats/index.html
- Motoyama YL, Pereira PEA, Esteves GDJ, Duarte JMP, Carrara VKP, Rissato GM, et al. Métodos alternativos para estimar a velocidade da máxima fase estável de lactato em adultos jovens fisicamente ativas. Rev Bras Cineantropom Desemp Hum. 2014;16(4):419. doi: 10.1590/1980-0037.2014v16n4p419 [Crossref]
- Rocha C, Canellas A, Monteiro D, Antoniazzi
M, Azevedo P. Changes in individual glucose threshold during military training.
Int J Sports Med. 2010;31(7):482-5. doi: 10.1055/s-0030-1248284 [Crossref]
- Sotero RC, Pardono E, Landwehr R, Campbell CSG, Simoes HG. Blood glucose minimum predicts maximal lactate steady state on running. Int J Sports Med. 2009 Sep 30;30(09):643-6. doi: 10.1055/s-0029-1220729 [Crossref]
- Weatherwax RM, Harris NK, Kilding
AE, Dalleck LC. Incidence of VO2max
responders to personalized versus standardized exercise prescription. Med Sci
Sport Exerc. [Internet]. 2019 [citado
2022 Fev 15];51(4):681-91. Available from:
http://journals.lww.com/00005768-201904000-00010
- Nilton T, Souza T, Alexandra S, Yamaguti L, Simões HG. Identificação do lactato mínimo e glicose mínima em indivíduos fisicamente ativos. Rev Bras Ciência e Mov. [Internet]. 2003 [citado 2022 Mar 3];71-5. Disponível em: https://pesquisa.bvsalud.org/portal/resource/pt/lil-524712
