Rev Bras Fisiol Exerc. 2022;21(6):365-80
doi: 10.33233/rbfex.v21i6.5411
REVIEW
Heart rate variability: a literature review on signal
recording, processing and interpretation, influencing factors, and
applicability to physical exercise
Variabilidade
da frequência cardíaca: uma revisão da literatura sobre o registro,
processamento e interpretação do sinal, fatores influenciadores, e
aplicabilidade ao exercício físico
Perciliany Martins de Souza1, Cássia
Regina Vieira Araújo1, Izabela Mocaiber2,
Carlos Eduardo Nórte3, Lenice Kappes Becker1, Gabriela Guerra Leal Souza1
1Universidade Federal de Ouro Preto, Ouro
Preto, MG, Brazil
2Universidade Federal Fluminense, RJ, Brazil
3Universidade do Estado do Rio de
Janeiro, Rio de Janeiro, RJ, Brazil
Received: August 10, 2022; Accepted: November 16,
2022.
Correspondence: Perciliany Martins de Souza, E-mail:
perciliany@yahoo.com.br
How to cite
Souza PM, Araújo CRV, Mocaiber
I, Nórte CE, Becker LK, Souza GGL. Heart rate
variability: a literature review on signal recording, processing and
interpretation, influencing factors, and applicability to physical exercise. Rev Bras
Fisiol Exerc. 21(6):365-80 doi: 10.33233/rbfex.v21i6.5411
Abstract
Aim: This narrative review sought to
address heart rate variability (HRV) based on concepts and definitions, methods
of recording and processing signals, signal interpretation, influencing
factors, and applicability to physical exercise. Methods: Bibliographic
survey of works published between 2000 and 2022 in PubMed, Scopus, Web of
Science, Scielo databases. Results: HRV is a
non-invasive method for measuring the action of the autonomic nervous system in
the heart and has been used as a marker of physical and mental health. HRV
measurements can be performed by recording an electrocardiogram or frequency
meter, which allows the extraction of several HRV parameters using linear and
non-linear methods. Conclusion: HRV is a variable that can be influenced
by several factors, and its role can be explained based on four theories.
Physical training can be used as a marker of exercise intensity control.
Keywords: autonomic nervous system; heart
rate variability; record; physical training; electrocardiogram.
Resumo
Objetivo: Esta revisão narrativa buscou abordar
a variabilidade da frequência cardíaca (VFC) em relação aos conceitos e
definições, formas de registro e processamento do sinal, interpretação do
sinal, fatores influenciadores e aplicações ao exercício. Métodos:
Levantamento bibliográfico de trabalhos publicados entre 2000 e 2022 nas bases
de dados PubMed, Scopus, Web of
Science, Scielo. Resultados: A VFC é um método
não invasivo de mensurar a atuação do sistema nervoso autônomo no coração, que
vem sendo utilizada como marcador de saúde física e mental. A mensuração da VFC
pode ser feita através do registro do eletrocardiograma ou frequencímetro, que
permitem a extração de diversos parâmetros da VFC utilizando métodos lineares e
não lineares. Conclusão: A VFC é uma variável que pode ser influenciada
por diversos fatores, e seu papel pode ser explicado com base em quatro
teorias. No que se refere ao treinamento físico, pode ser usada como um
marcador de controle de intensidade de exercício.
Palavras-chave: sistema nervoso autônomo;
variabilidade da frequência cardíaca; registro; treinamento físico;
eletrocardiograma.
Introduction
The autonomic nervous system (ANS)
modulates the heart by regulating its extrinsic rhythm, thereby influencing the
frequency of contractions [1]. Communication between the ANS and heart is
dependent on information from mechanoreceptors (cardiopulmonary and
baroreceptors) and chemoreceptors (carotid, aortic, and ventricular).
Influences derived from the respiratory, vasomotor,
renin–angiotensin–aldosterone, and thermoregulatory systems and from higher
centers, such as the amygdala and hypothalamus, reach the medullary
cardiovascular centers, which modulate sympathetic and parasympathetic (vagal)
autonomic activity in the heart [2].
Autonomic pathways are formed by
preganglionic and postganglionic fibers. The parasympathetic system is composed
of neurons that originate in the medullary nuclei (more specifically, in the
dorsal motor nucleus of the vagus and nucleus ambiguus), with preganglionic fibers, which have elongated
axons and establish synapses with postganglionic neurons, which have short
axons located in the cardiac plexus and reach the cardiac muscle through the
sinus and atrioventricular nodes [3]. With the stimulation of the
parasympathetic ANS, acetylcholine is released by the preganglionic and postganglionic
fibers, reducing the sinus node firing rate, cardiac output, and blood
pressure. Generally, this type of stimulation is predominant in situations of
rest, safety, and digestion [4].
Sympathetic pathways are formed by
fibers originating in the medulla (more specifically, in the reticular
formation) with projections to preganglionic fibers located in the thoracic
spinal cord that synapse with postganglionic fibers located in the
paravertebral ganglia, finally reaching the sinus node and distributing to the
atrioventricular node and most of the myocardium. Upon stimulation of the
sympathetic ANS, norepinephrine is released, which increases the sinus node
firing rate, electrical conduction velocity, excitability, and contraction
force in all portions of the heart. Therefore, cardiac output and ejection
volume increase, thus increasing the blood pressure [3]. An overview of the
anatomy of the sympathetic and parasympathetic pathways is shown in Figure 1.
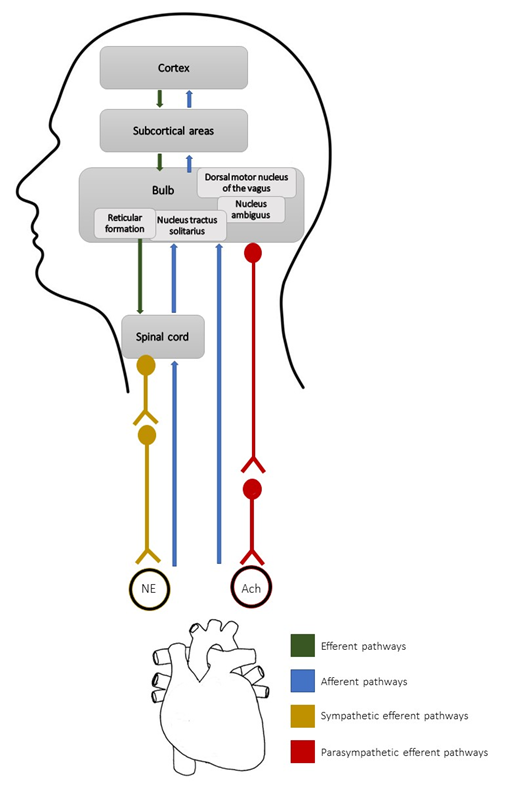
NE = norepinephrine; Ach =
acetylcholine. Source: author himself
Figure 1 - Communication pathways
between the brain and the heart, which are responsible for generating heart
rate variability
The signaling mechanisms of the
sympathetic and parasympathetic branches, as well as their temporal courses,
are distinct. Sympathetic influence on the heart has a slower course of action
than parasympathetic influence. Changes in heart rate (HR) resulting from
sympathetic activation occur more slowly, with a peak effect observed
approximately 4 s after the start of stimulation and a return to baseline
occurring approximately 20 s after the cessation of stimulation. In contrast,
parasympathetic modulation on the heart has low response latency, with a peak
effect within 0.5 s after the start of stimulation and a return to baseline
within 1 s after cessation. Thus, the parasympathetic modulation of the heart
occurs faster than sympathetic modulation. These differences in the time of
action occur because of the different types of intracellular signaling
mechanisms underlying different receptors. Sympathetic activity occurs through
the action of noradrenaline on β1-adrenergic receptors, which generates a
cascade of intracellular reactions in multiple stages, culminating in the
opening of Na+ and Ca2+ channels and closing of K+
channels. Owing to these stages, sympathetic activity generates slow
fluctuations in the heart (not greater than 0.15 Hz in the interval between
heartbeats). However, parasympathetic activity occurs through the action of
acetylcholine on metabotropic muscarinic receptors (M2), which opens the K+
and Ca2+ channels. Changes are initiated 30-100 ms
after binding of acetylcholine to its receptor. Thus, vagal activity can
generate high-frequency fluctuations in the interval between heartbeats between
0.15 and 0.4 Hz [5]. Therefore, it is understood that the HR variations are
determined by the integration between the slow and fast modulation generated by
the sympathetic and parasympathetic branches, respectively [6,7,8].
Stimulation arising from
sympathetic and parasympathetic modulation reaches the heart and can be studied
by recording the electrocardiograms, represented by waves P, Q, R, S, T, and U
(Figure 2A). Cardiac excitation begins with an impulse generated by
autorhythmic cells in the sinus node, which is distributed by the atrial
syncytium, resulting in the onset of depolarization of the atria (P wave),
followed by the complete depolarization of the atria (Q wave). This impulse
quickly reaches the atrioventricular node and is conducted from the
atrioventricular node to the ventricles by the atrioventricular bundle and
Purkinje fibers, resulting in atrial repolarization and the onset of
ventricular depolarization (R wave), followed by complete depolarization of the
ventricle (S wave), by the onset by ventricular repolarization (T wave), and
finally by ventricular repolarization (U wave) [9]. The demarcation of each of
these moments is shown in the electrocardiogram graph (Figure 2B).
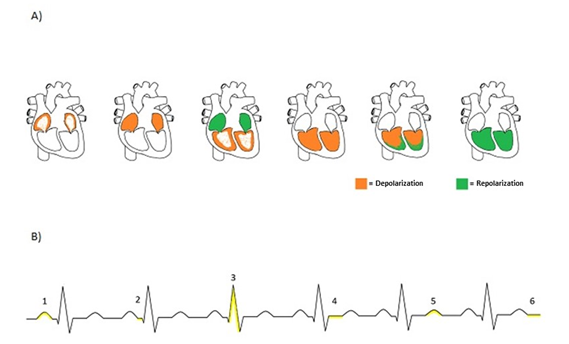
Source: author himself
Figure 2 - (A) Cardiac depolarization
and repolarization; (B) Electrocardiogram. Where 1 is the beginning of atrial
depolarization, 2 is complete atrial depolarization, 3 is the beginning of
ventricular depolarization and atrial repolarization, 4 is complete ventricular
depolarization, 5 is the beginning of repolarization, and 6 is complete
repolarization
The interval between two
ventricular depolarizations that generate actual heartbeats is known as the RR
interval [10]. From the time intervals between two R waves, in milliseconds, a
graph of the intervals between heartbeats over time can be constructed [1],
known as a tachogram.
The intervals between R waves on
the electrocardiogram are not regular. This variation in the duration of RR
intervals is healthy and expected, demonstrating that the heart does not
function like a metronome [9]. According to Shaffer et al. [9], the
greater the differences between these intervals, the greater the indications of
homeostasis, health, and physiological regulation of the individual in the face
of environmental demands. Physiological variation measured from the temporal
distance between RR intervals is known as heart rate variability (HRV). HRV is
a non-invasive and efficient method for assessing the activity of the ANS, that
is, the sympathetic and parasympathetic branches, in the heart [11].
After this initial
contextualization, the objective of the present study was to provide detailed
and updated information about HRV in relation to concepts and definitions;
forms of recording, processing, and interpretation of the signal; influencing
factors; and applications to reach people who are starting to study the
subject.
Methods
As this is a narrative review, the methodology used was based on a bibliographical survey of the subject in question conducted on different research platforms (PubMed, Scopus, Web of Science, and Scielo). The bibliographic survey covered, in almost its entirety, works published between 2000 and 2022. Notably, narrative reviews are broad research methods suitable for describing and discussing the development of a given subject from a theoretical or contextual point of view.
Results and Discussion
Registration and signal processing
According to the European Society
of Cardiology and North American Society of Electrophysiology [11], HRV
analysis can be performed using linear and non-linear methods. Linear methods
span both time and frequency domains. Frequency-domain analysis, also known as
spectral analysis, is capable of decomposing the tachogram
power spectrum into various frequency components or modulation rates of the RR
intervals (Table I).
The quoted frequency bands can be
calculated in absolute units (ms²) or normalized units (n.u.).
In normalized units, the low- (LF) and high-frequency (HF) bands are calculated
as a percentage of the total power, disregarding the very-low-frequency (VLF)
power:
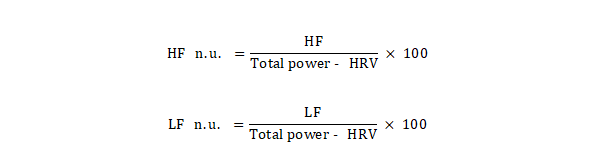
This normalization minimizes the
effects of changes in the VLF range on the two fast frequency ranges (LF and
HF), leaving only the purest effects of LF and HF [11].
In the analysis of HRV through the
time domain, each normal RR interval is measured, that is, the sinus beats
during a certain time interval; then, based on statistical or geometric methods
(mean, standard deviation, and derived indices from the histogram or the map of
Cartesian coordinates of the RR intervals), the indices that translate
fluctuations in the duration of the cardiac cycles are calculated, thus
providing several parameters (Chart 1).
Chart I - Linear methods of HRV analysis
The non-linear method has gained
visibility in recent years, and perhaps for this reason, the physiological
interpretation of its indices is still not well defined. Several studies have
indicated possible physiological interpretations: i) Poincaré plot: it has been proposed that the component
standard deviation 1 (SD1) represents the parasympathetic activity, the SD2
represents the sympathetic activity and parasympathetic, while the SD1/SD2
ratio represents the sympathetic and parasympathetic ratio [17]; ii) Detrended
Fluctuation Analysis (DFA): the proposal is that the short-term correlations
extracted with DFA (α1) reflect the baroreceptor reflex, while the
long-term correlations (α2) reflect the regulatory mechanisms that limit
the fluctuation of the beating cycle [18]. In addition to these two parameters,
the non-linear method comprises different methods for processing HRV. Some of
these forms are listed in Table II and are adapted from Ferreira et al.
[7].
Chart II - Non-linear HRV analysis
methods
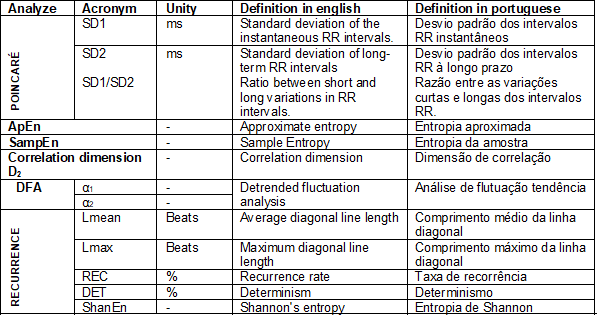
Among all the non-linear methods
mentioned, the Poincaré plot method or the Poincaré plot is highly detailed, as this is the most
studied method in the literature. This is the analysis of a scatter plot, where
each point is represented on the x-axis as RRn and
the y-axis as RRn+1, where RRn is the time between
two successive R waves, and RRn+1 is the time between two successive R peaks.
When the plot is fitted using the ellipse-fitting method, the analysis provided
three indices: the SD of instantaneous beat-to-beat interval variability (SD1),
long-term variability of continuous RR intervals (SD2), and SD1/SD2. In the Poincaré plot, SD1 is the width, and SD2 is the length of
the ellipse. It is also possible to extract the area of an imaginary ellipse (S)
using the length axes (S = SD1 × SD2) [19,20,21].
A Poincaré
plot can be analyzed qualitatively or quantitatively. Qualitatively, the
analysis is performed by observing the shape of the cloud of points and,
consequently, the ellipse. A graph with a very concentrated cloud of points may
be indicative of some pathology; however, high dispersions in the time series
may indicate good health [22,23,24].
For the quantitative analysis of
the Poincaré plot, SD1 reflects the instantaneous
beat-to-beat variability of HR and represents parasympathetic activity, and SD2
reflects the continuous variability from beat-to-beat and represents the global
HRV and sympathetic and parasympathetic activity [22,25].
Interpretation of the HRV signal
There are currently some theories
that attempt to explain the evolutionary role of HRV, among which four stand
out: i) polyvagal theory [26,27], ii) behavioral
biological model [28], iii) neurovisceral integration model [29], and iv)
respiratory rate resonance model [30,31].
According to the polyvagal theory
developed by Porges in 1995, during the evolution of
mammals, anatomical and physiological changes occur in the ANS, which are
responsible for influencing HRV in these animals. One of these changes was the
myelination of the motor fibers of the vagus nerve
originating in the nucleus ambiguus, which provided
rapid modulation of the HRV that occurs in contexts where the animal is safe,
facilitating affiliative behaviors, care for the offspring, and social approach
[26,27].
Grossman and Taylor [28] developed
a behavioral biological model, a highly comprehensive alternative that
emphasizes the primary function of HRV as synchronization between the
respiratory and cardiovascular systems, enabling energy conservation by
optimizing the efficiency of gas exchange. This model emphasizes that HRV is
not necessarily a reliable measure of vagal activity generated by the nucleus ambiguus in the SA node of the heart but may reflect a wide
variety of physiological and behavioral changes in the body with an
energy-saving function.
The neurovisceral integration model
developed by Thayer and Lane [29] uses HRV as an index of integration between
the central nervous system and ANS. This model associates individual
differences in cognitive performance, especially executive function, with HRV,
particularly in aspects mediated by the vagus nerve.
This suggests that HRV may serve as a peripheral index of central nervous
system integrity, which aids goal-directed behavior. In the security context,
subcortical circuits are inhibited via prefrontal cortical circuits, allowing
for an increase in HRV and an improvement in executive function. In contrast,
in contexts of threat, uncertainty or novelty, subcortical circuits are
activated due to prefrontal hypoactivation; thus, sympathoexcitatory
preparation for action occurs, resulting in a decrease in HRV.
Lehrer and Gevirtz
[31] proposed a respiratory rate resonance model that emphasized the influence
of breathing on HRV by modulating vagal and baroreflex activities. An important
practical implication derived from this model is that the HRV of individuals
can be maximized at individualized respiratory rates, which can be stimulated
through cardiorespiratory biofeedback training [30,31].
These theories encompass different
aspects of HRV, which provide considerable enrichment for the interpretation of
changes in different HRV parameters, especially parasympathetic parameters, in
experimental contexts and the expansion of perspectives in several research
fields that use this research tool.
Factors that can influence HRV
HRV is influenced by several
factors. Owing to the diversity of factors, this review presents the most
studied factors in the literature: types and intensity of physical exercises
practiced regularly and/or during electrocardiogram collection, cognitive
tasks, anthropometric variables, sex, age, circadian rhythm, breathing
(frequency and amplitude), food, hydration, nicotine, drugs, and physical
conditioning. As such factors affect HRV and can weaken the results of the
studies, it is recommended to eliminate them or to control them statistically,
regardless of the chosen method of signal processing (linear or non-linear).
Anthropometric variables (body mass,
body mass index [BMI], waist and hip circumference, and fat percentage) can
influence HRV. Epidemiological studies have shown that poor anthropometric
indicators, such as high waist circumference, visceral fat area, and BMI, can
be negatively associated with HRV components, especially those related to
parasympathetic activity [32,33].
Sex and age can also influence HRV.
Xhyheri et al. [34] showed that healthy women
have lower HRV values than healthy men with increasing age, owing to decreased
estrogen release in women. There is a tendency for HRV to decrease in both
sexes, with differences between sexes, and disappear with age. This has been
expounded in several other studies [35,36,37,38].
Regarding the circadian rhythm, it
is possible to observe that the HRV increases during the night and decreases
during the day [39]. After evaluating 26 original studies, Sammito
et al. [40] pointed out that almost all studies detected that the
circadian rhythm influenced the HRV parameters analyzed and that the HRV
increased during the night, with a peak identified during the second half of
the night. More specifically, Li et al. [41] proposed that HRV is higher
at night and decreases at approximately 10 am, remaining low until 7 pm, when
it rises again.
Another very important variable to
mention is breathing. HR increases during the inspiration phase and decreases
during the expiration phase [42]. This effect occurs because of the inhibition
of parasympathetic discharge in the cardiovascular center during inspiration,
causing the HR to increase. During inspiration, there is a decrease in vagal
activity, which causes tachycardia; during expiration, there is an increase in
activity, which causes bradycardia. This physiological alteration is called
Respiratory Sinus Arrhythmia and leads to variations in HR due to breathing.
Thus, there is a predominance of activation of the parasympathetic ANS (during
expiration), mainly responsible for the ASR. As the respiratory rhythm during
rest is approximately 0.25 Hz, approximately 15 inspirations and expirations
per minute, and the frequency of the HRV HF band is between 0.15 and 0.4, it is
inferred that there is a correspondence between the ASR and the HF component of
HRV. Most power in the HF band is generated by respiration [43]. Therefore, HF
represents ASR, and as ASR occurs due to the activity of the parasympathetic
ANS, HF represents cardiac parasympathetic modulation [42]. Thus, given that
breathing influences HRV, there have been several proposals for its measurement
or control during HRV recording [17,44].
Food and liquid intake are rarely
considered in research using HRV; however, both deserve attention. For example,
meals of 500 kcal can reduce the vagal parameters of HRV even after an hour of
ingestion [45]. In contrast, fasting for long periods may increase HRV vagal
activity [46,47]. Therefore, it is recommended that research volunteers consume
a light meal approximately 2 h before HRV recording [48]. Water consumption can
also reduce HRV, particularly in the HF component [49], owing to a vagal
tamponade response to the pressor effect caused by hypoosmotic fluids [50].
Other authors also support the idea that bladder and stomach distention can
reduce HRV [51,52]. Therefore, it is recommended that volunteers empty their
bladders immediately before the experiment [48,53].
Another important point to be
controlled and studied in the literature is nicotine consumption. Nicotine
consumption is associated with a reduction in the activity of the
parasympathetic nervous system [54], and in some studies, this goes beyond this
statement. Arastoo et al. [54], for example,
evaluated 100 smokers, including 58 chronic e-cigarette users and 42 chronic
tobacco cigarette smokers, and observed that both types of chronic smokers
exhibited a similar baseline HRV pattern, which consisted of a reduction in the
components of parasympathetic compared with non-smokers.
There is also a relationship
between medication use and changes in HRV. Several drugs, especially antidepressants,
antipsychotics, and antihypertensives, cause a reduction in HRV [55,56,57]. Among
psychotropic medications, a systematic review revealed that only tricyclic
antidepressants and clozapine statistically influenced HRV [58]. However, it is
recommended to document and, when possible, exclude volunteers who use any
medication that acts on the nervous, cardiovascular, and respiratory systems.
Finally, during the practice of
physical exercise, it is expected that the HRV parameters associated with vagal
activity decrease and, after its end, return to resting levels, which is
considered an expected, flexible, and adaptive autonomic response [59,60].
Notably, during exercise, the intensity can produce different responses that
vary according to the level of effort. Maximum or submaximal physical exertion,
for example, may influence vagal withdrawal differently [61]. Vagal activity
decreases progressively until complete withdrawal at approximately 50-60% of
peak oxygen consumption for maximal exercise protocols, and this
intensity-differentiated response extends for more than 10 min after the end of
exercise [62]. Another factor is the level of fitness and endurance [62].
Individuals with low levels of physical conditioning have high sympathetic
activity and low parasympathetic activity at rest [63].
Applicability to physical exercise
Therefore, the cardioprotective
effects of physical exercise are undeniable. However, acutely, especially at
high intensities, it is considered a stressful stimulus that increases the risk
of lethal arrhythmias [64,65]. Thus, cardiac autonomic changes, such as
parasympathetic (vagal) reduction and sympathetic increase, which occur during
physical exercise, create a situation conducive to the development of
ventricular ectopic activities, which can culminate in cardiac arrest or sudden
death of an individual [66]. Based on this, Albert et al. [65] proposed
the schematic model of the “window of exposure” to cardiovascular risks.
According to them, owing to the unfavorable autonomic state during and a few
minutes immediately after physical exercise, the practitioner is exposed to
very high cardiovascular risks, which only cease when the autonomic parameters
are reestablished at resting values. Nevertheless, regular physical exercise is
one way to improve vagal flexibility, which is defined as the ability of the
ANS to respond to a given stimulus [67].
Although HRV at rest has been well
studied, its evaluation during exercise is still not completely understood,
mainly because of the inconsistent results of HRV during exercise, such as the
lack of vagal withdrawal during a physical test. Such conditions may be due to
the variety of methods and protocols (maximum and submaximal efforts) used
[68,69,70,71], physical fitness levels, resistance, body composition, and sex
differences [62,63]. In general, it is thought that during physical exercise,
HRV decreases relative to rest and returns to baseline values during recovery.
In addition, during exercise, there is a gradual decrease in parasympathetic
activity and an increase, also progressive, in sympathetic stimulation, which
may be related to the increase in exercise intensity [72].
Among the possible HRV influencers
listed above, we reinforce the idea that individuals with low levels of
physical fitness have high sympathetic activity and low parasympathetic
activity at rest, which is a negative effect, especially when evaluated under
the influence of physical exercise [63]. As a result, the resting HRV of these
individuals is lower, and the “window of exposure” to cardiovascular risks is
greater, that is, they take longer to recover from exercise.
During exercise, intensity can
produce different responses, varying according to effort level and sex. Vagal
activity decreases progressively until its complete withdrawal at approximately
50-60% of peak maximal oxygen consumption for maximum protocols, and this
response, differentiated by intensity, extends for more than 10 minutes after
the end of the exercise [62,63]. Finally, we also point out that HRV components
can be related (inversely proportional relationship) to the lactate threshold
or ventilatory thresholds 1 and 2, which are important parameters for measuring
or controlling the intensity of physical exercise [73,74,75].
Conclusion
Considering that heart rate
variability is a low-cost, easy-to-acquire, and noninvasive parameter for
assessing the ANS, this review aimed to provide researchers with an overview of
the different issues that permeate the use of HRV parameters. It is worth
mentioning that these recommendations seek to standardize HRV recording and
processing and identify the influencing factors for researchers to plan their
experiments in the most adequate way possible so that the results faithfully
represent the changes in HRV components and are not affected by confounding
variables. Finally, the interpretation of HRV is important for providing
meaning and relevance to studies in this area. Given that HRV has been
consistently identified as a marker of physical and mental health, public
health policies are expected to be implemented in the future to allow routine
use of this index by multidisciplinary teams, especially during physical
exercise. Finally, we emphasize that the HRV is an important means for controlling
the intensity of the exercise, including with regards to the cost-benefit, such
as the devices necessary for assessing the ventilatory threshold and lactate.
Academic attachment
This article represents part of the
literature review used in the thesis by Perciliany
Martins de Souza, supervised by Professor Ph. D. Gabriela Guerra Leal Souza,
and co-supervised by Dr. Eduardo Bearzoti at the
Federal University of Ouro Preto, Ouro
Preto.
Conflict of interests
The authors declare that there is
no conflict of interest.
Funding
This work was supported by the
Coordination for the Improvement of Higher Education Personnel, Brazil (CAPES);
National Council for Scientific and Technological Development, Brazil (CNPq); Research Support Foundation of the State of Minas
Gerais, Brazil (FAPEMIG); and the Federal University of Ouro
Preto, Brazil (UFOP).
Authors' contributions
Research conception: Souza PM and Souza GGL; Funding: Souza PM and Souza GGL, Writing
of the manuscript: Souza PM, Araújo CRV, Mocaiber
I, North CE, Becker LK, and Souza GGL; Critical review of the manuscript for
important intellectual content: Becker LK and Souza GGL.
References
- Sztajzel
J. Heart rate variability: a noninvasive electrocardiographic method to measure
the autonomic nervous system. Swiss Med Wkly. 2004;134(35-36):514-22. doi: 10.4414/smw.2004.10321 [Crossref]
- Berntson GG, Bechara A, Damasio H, Tranel D, Cacioppo JT. Amygdala contribution to selective dimensions of emotion. Soc Cogn Affect Neurosci. 2007;2(2):123-9. doi: 10.1093/scan/nsm008 [Crossref]
- Koeppen
BM, Stanton BA. Berne y Levy. Fisiología+ StudentConsult. Spain: Elsevier
Health Sciences; 2009.
- Brownley
K, Hurwitz B, Schneiderman N. Cardiovascular Psychophysiology. In: Cacioppo T,
Tassinary LG, Berntson GG, eds. Handbook of Psychophysiology. Cambridge, UK:
Cambridge University Press; 2000. p. 224-64.
- Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J Physiol Anthropol. 2019;38(1):1-8. doi: 10.1186/s40101-019-0193-2 [Crossref]
- Cambri
LT, Fronchetti L, Gevaerd MS. Variabilidade
da frequência cardíaca e controle metabólico. Arq Sanny Pesq Saúde.
2008;1(1):72-82.
- Ferreira M, Messias M, Vandereli LCM, Pastre CM. Caracterização do comportamento caótico da variabilidade da frequência cardíaca (VFC) em jovens saudáveis. Trends in Applied and Computational Mathematics. 2010;11(2):50. doi: 10.5540/tema.2010.011.02.0141 [Crossref]
- Fronchetti
L, Nakamura FY, Lima-Silva AE, Lima JRP. Effects of high-intensity interval
training on heart rate variability during exercise. J Exerc Physiol Online
[Internet]. 2007[cited 2022 Jan 2];10(4):1-9. Available from:
https://www.researchgate.net/publication/257874986_Effects_of_high-intensity_interval_training_on_heart_rate_variability_during_exercise
- Shaffer FR, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040 [Crossref]
- Bae TW, Kwon KK. ECG PQRST complex detector and heart rate variability analysis using temporal characteristics of fiducial points. Biomed Signal Process Control. 2021;66:102291. doi: 10.1016/j.bspc.2020.102291 [Crossref]
- Task
F. Heart rate variability. Standards of measurement, physiological
interpretation, and clinical use. Eur
Heart J. 1996;17(3):354-81.
- Barbosa MPR, Netto Junior J, Cassemiro
BM, Bernardo AFB, Silva AKB, Silva AKF, Vanderlei, et al. Effects
of functional training on geometric indices of heart rate variability. J Sport
Health. Sci 2016;5(2):183-9. doi: 10.1016/j.jshs.2014.12.007 [Crossref]
- Ribeiro
JP, Moraes Filho RS. Variabilidade
da frequência como instrumento de investigação do sistema nervoso
autônomo. Rev Bras Hipertens. 2005;25(3)14-20.
- Goldstein DS, Bentho O, Park M, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol 2011;96(12):1255-61. doi: 10.1113/expphysiol.2010.056259 [Crossref]
- Billman GE.The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013;4:26. doi: 10.3389/fphys.2013.00026 [Crossref]
- Kim JB, Seo BS, Kim JH. Effect of arousal on sympathetic overactivity in patients with obstructive sleep apnea. Sleep Med. 2019;62:86-91. doi: 10.1016/j.sleep.2019.01.044 [Crossref]
- Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;8:213. doi: 10.3389/fpsyg.2017.00213 [Crossref]
- Shaffer F, Ginsberg J. An overview of heart rate variability metrics and norms. Frontiers in Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258 [Crossref]
- Guzik P, Piskorski J, Krauze T, Schneider R, Wesseling KH, Wykretowicz A, Wysocki H. Correlations between the Poincaré plot and conventional heart rate variability parameters assessed during paced breathing. J Physiol Sci 2007;57(1):63-71. doi: 10.2170/physiolsci.RP005506 [Crossref]
- Quintana DS, Heathers JA. Considerations in the assessment of heart rate variability in biobehavioral research. Front Psychol. 2014;5:805. doi: 10.3389/fpsyg.2014.00805 [Crossref]
- Vanderlei, L.C.M., Pastre CM, Freitas Junior IF, Godoy MF. Índices geométricos de variabilidade da frequência cardíaca em crianças obesas e eutróficas. Arq Bras Cardiol. 2010;95(1):35-40. doi: 10.1590/S0066-782X2010005000082 [Crossref]
- Acharya UR, Joseph PK, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006;44(12):1031-51. doi: 10.1007/s11517-006-0119-0 [Crossref]
- Kitlas
A, Oczeretko E, Kowalewski M, Borowska M, Urban M. Nonlinear dynamics methods
in the analysis of the heart rate variability. Annales Academicae Medicae
Bialostocensis. 2005;50(Suppl 2):46-7.
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4(6):481. doi: 10.1038/nrn1118 [Crossref]
- Lombardi
F. Chaos theory, heart rate variability, and arrhythmic mortality. Circulation.
2000;101:8-10. doi: 10.1161/01.cir.101.1.8 [Crossref]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32(4):301-18. doi: 10.1111/j.1469-8986.1995.tb01213.x [Crossref]
- Porges SW. The polyvagal theory: Phylogenetic contributions to social behavior. Physiol Behav. 2003;79(3):503-13. doi: 10.1016/s0031-9384(03)00156-2 [Crossref]
- Grossman
P,Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to
cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol.
2007;74(2):263-85.
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201-16. doi: 10.1016/s0165-0327(00)00338-4 [Crossref]
- Lehrer P, Kaur K, Sharma A, Shah K. Heart rate variability biofeedback improves emotional and physical health and performance: a systematic review and meta analysis. Appl Psychophysiol Biofeedback. 2020;45:109-29. doi: 10.1007/s10484-020-09466-z [Crossref]
- Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Front Psychol. 2014;5:756. doi: 10.3389/fpsyg.2014.00756 [Crossref]
- Monteze NM, Souza BB, Alves HJP, Oliveira FLP, Oliveira JM, Freitas SN, et al. Heart rate variability in shift workers: responses to orthostatism and relationships with anthropometry, body composition, and blood pressure. Biomed Res Int. 2015;2015:329057. doi: 10.1155/2015/329057 [Crossref]
- Koenig J, Jarczok MN, Warth M, Ellis RJ, Bach C, Hillecke TK, et al. Body mass index is related to autonomic nervous system activity as measured by heart rate variability - a replication using short term measurements. J Nutr Health Aging. 2014:1-3. doi: 10.1007/s12603-014-0022-6 [Crossref]
- Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R, et al. Heart rate variability today. Prog Cardiovasc Dis. 2012;55(3):321-31. doi: 10.1016/j.pcad.2012.09 [Crossref]
- Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci Biobehav Rev. 2016;64:288-310. doi: 10.1016/j.neubiorev.2016.03.007 [Crossref]
- Sammito S, Böckelmann I. Reference values for time-and frequency-domain heart rate variability measures. Heart Rhythm. 2016;13(6):1309-16. doi: 10.1016/j.hrthm.2016.02.006 [Crossref]
- Almeida-Santos MA, Barreto-Filho JA, Oliveira JLM, Reis FP, Oliveira CCC, Sousa ACS. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch Gerontol Geriatr. 2016;63:1-8. doi: 10.1016/j.archger.2015.11.011 [Crossref]
- Nunan D, Sandercock GR, Brodie DA. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol 2010;33(11):1407-17. doi: 10.1111/j.1540-8159.2010.02841.x [Crossref]
- Hayano
J, Yasuma F. Hypothesis: respiratory sinus arrhythmia is an intrinsic resting
function of cardiopulmonary system. Cardiovasc Res. 2003;58(1):1-9. doi: 10.1016/s0008-6363(02)00851-9 [Crossref]
- Sammito S, Sammito W, Böckelmann I. The circadian rhythm of heart rate variability. Biological Rhythm Research. 2016;47(5):717-30. doi: 10.1080/09291016.2016.1183887 [Crossref]
- Li X, Shaffer ML, Rodriguez-Colon S, He F, Wolbrette DL, Alagona Jr P, et al. The circadian pattern of cardiac autonomic modulation in a middle-aged population. Clin Auton Res. 2011;21(3):143-50. doi: 10.1007/s10286-010-0112-4 [Crossref]
- Yasuma F, Hayano JI. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125(2):683-90. doi: 10.1378/chest.125.2.683 [Crossref]
- Berntson GG, Bigger Junior T, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623-48. doi: 10.1111/j.1469-8986.1997.tb02140.x [Crossref]
- Quintana D, Alvares GA, Heathers J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry. 2016;6(5):e803-e803. doi: 10.1038/tp.2016.73 [Crossref]
- Lu CL., Zou X, Orr WC, Chen JD. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig Dis Sci. 1999;44(4):857-61. doi: 10.1023/a:1026698800742 [Crossref]
- Cansel M, Taşolar H, Yağmur J, Ermiş N, Açıkgöz N, Eyyüpkoca F, et al. The effects of Ramadan fasting on heart rate variability in healthy individuals: A prospective study. Anadolu Kardiyol Derg. 2014;14(5). doi: 10.5152/akd.2014.5108 [Crossref]
- Pivik R, Dykman RA, Tennal K, Y Gu Y. Skipping breakfast: gender effects on resting heart rate measures in preadolescents. Physiol Behav. 2006;89(2):270-80. doi: 10.1016/j.physbeh.2006.06.001 [Crossref]
- Tak LM, Riese H, Bock GH, Manoharan A, Kok IC, Rosmalen JGM. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol Psychol. 2009;82(2):101-10. doi: 10.1016/j.biopsycho.2009.05.002 [Crossref]
- Routledge HC, Chowdhary S, Coote JH, Townend JN. Cardiac vagal response to water ingestion in normal human subjects. Clin Sci. 2002;103(2):157-62. doi: 10.1042/cs1030157 [Crossref]
- Scott
EM, Greenwood JP, Gilbey SG, Stoker JB, Mary DA. Water ingestion increases
sympathetic vasoconstrictor discharge in normal human subjects. Clin Sci
2001;100(3):335-42.
- Fagius J, Karhuvaara S. Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension. 1989;14(5):511-17. doi: 10.1161/01.hyp.14.5.511 [Crossref]
- Rossi P, Andriesse GI, Oey PL, Wieneke GH, Roelofs JM, Akkermans LM. Stomach distension increases efferent muscle sympathetic nerve activity and blood pressure in healthy humans. J Neurol Sci. 1998;161(2):148-55. doi: 10.1016/s0022-510x(98)00276-7 [Crossref]
- Heathers JA, Everything Hertz: methodological issues in short-term frequency-domain HRV. Front Physiol. 2014;5:177. doi: 10.3389/fphys.2014.00177 [Crossref]
- Arastoo S, Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, et al. Acute and chronic sympathomimetic effects of e-cigarette and tobacco cigarette smoking: role of nicotine and non-nicotine constituents. Am J Physiol Heart Circ Physiol. 2020;319(2):H262-H270. doi: 10.1152/ajpheart.00192.2020 [Crossref]
- Cacciotti-Saija C, Quintana DS, Alvares GA, Hickie IB, Guastella AJ. Reduced heart rate variability in a treatment-seeking early psychosis sample. Psych Res. 2018;269:293-300. doi: 10.1016/j.psychres.2018.08.068 [Crossref]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67(11):1067-74. doi: 10.1016/j.biopsych.2009.12.012 [Crossref]
- Maciorowska M, Krzesiński P, Wierzbowski R, Gielerak G. Heart rate variability in patients with hypertension: the effect of metabolic syndrome and antihypertensive treatment. Cardiovasc Ther. 2020. doi: 10.1155/2020/8563135 [Crossref]
- Alvares GA, Quintana DS, Hickie IB, Guastella AJ. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016. doi: 10.1503/jpn.140217 [Crossref]
- Souza PM, Rosário NSA, Pinto KMC, Assunção PE, Oliveira FLP, Eduardo Bearzoti E. Vagal flexibility during exercise: impact of training, stress, anthropometric measures, and gender. Rehabil Res Pract. 2020: 6387839. doi: 10.1155/2020/6387839 [Crossref]
- Marasingha-Arachchige SU, Rubio-Arias JA, Alcaraz PE, Chung LH. Factors that affect heart rate variability following acute resistance exercise: A systematic review and meta-analysis. J Sport Health Sci. 2022;11(3):376-92. doi: 10.1016/j.jshs.2020.11.008 [Crossref]
- Mourot L, Bouhaddi M, Perrey S, Rouillon JD, Regnard J. Quantitative Poincaré plot analysis of heart rate variability: effect of endurance training. Eur J Appl Physiol. 2004;91(1):79-87. doi: 10.1007/s00421-003-0917-0 [Crossref]
- Michael S, Jay O, Halaki M, Graham K, Davis GM. Submaximal exercise intensity modulates acute post-exercise heart rate variability. Eur J Appl Physiol. 2016;116(4):697-706. doi: 10.1007/s00421-016-3327-9 [Crossref]
- Tulppo MP, Mäkikallio TH, Seppänen T, Airaksinen JK, Huikuri HV. Heart rate dynamics during accentuated sympathovagal interaction. Am J Physiol. 1998;274(3 Pt 2):H810-6. doi: 10.1152/ajpheart.1998.274.3.H810 [Crossref]
- Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiology Clinics. 1996;14(2):263-70. doi: 10.1016/s0733-8651(05)70279-4 [Crossref]
- Albert CM, Mattana J. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343(19):1355-61. doi: 10.1056/NEJM200011093431902 [Crossref]
- Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training--what's the link? Exp Physiol. 2002;87(4):423-35. doi: 10.1111/j.1469-445x.2002.tb00055.x [Crossref]
- Billman GE. Aerobic exercise conditioning: a nonpharmacological antiarrhythmic intervention. J Appl Physiol. 2002;92(2):446-54. doi: 10.1152/japplphysiol.00874.2001 [Crossref]
- Kamath
MV, Fallen EL, McKelvie R. Effects of steady state exercise on the power
spectrum of heart rate variability. Med Sci Sports Exerc. 1991;23(4):428-34.
- Yamamoto Y, Hughson RL, Peterson JC. Autonomic control of heart rate during exercise studied by heart rate variability spectral analysis. J Appl Physiol. 1991;71(3):1136-42. doi: 10.1152/jappl.1991.71.3.1136 [Crossref]
- Tulppo MP, Mäkikallio TH, Takala TE, Seppänen T, Huikuri HV. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol. 1996;271(1 Pt 2):H244-52. doi: 10.1152/ajpheart.1996.271.1.H244 [Crossref]
- Mourot L, Bouhaddi M, Perrey S, Rouillon JD, Regnard J. Quantitative Poincare plot analysis of heart rate variability: effect of endurance training. Eur J Appl Physiol. 2004;91(1):79-87. doi: 10.1007/s00421-003-0917-0 [Crossref]
- Kiviniemi AM, Hautala AJ, Mäkikallio TH, Seppänen T, Huikuri HV, Tulppo MP. Cardiac vagal outflow after aerobic training by analysis of high-frequency oscillation of the R-R interval. Eur J Appl Physiol. 2006;96(6):686-92. doi: 10.1007/s00421-005-0130-4 [Crossref]
- Nascimento EMF, Kiss MAPD, Santos TM, Lambert M, Pires FO. Determination of lactate thresholds in maximal running test by heart rate variability data set. Asian Journal of Sports Medicine. 2017;8(3). doi: 10.5812/asjsm.58480 [Crossref]
- Nascimento EMF, Antunes D, Salvador PCN, Borszcz FK, Lucas RD. Applicability of Dmax method on heart rate variability to estimate the lactate thresholds in male runners. J Sports Med. 2019. doi: 10.1155/2019/2075371 [Crossref]
- Scherer M, Martinek J, Mayr W. HRV (Heart Rate Variability) as a non-invasive measurement method for performance diagnostics and training control. Curr Dir Biomed Eng. 2019;5(1):97-100. doi: 10.1515/cdbme-2019-0025 [Crossref]
