Rev Bras Fisiol Exerc. 2023;22:e225468
doi: 10.33233/rbfex.v22i1.5468
REVIEW
Protocol to reduce the risk of unintentional doping caused by dietary supplements ingestion
Protocolo de redução de risco de doping não intencional causado por consumo de suplementos alimentares
Renata
Rebello Mendes1, João Rafael Santos Prado1
1Universidade Federal de Sergipe,
Aracaju, SE, Brazil
Received: May 5, 2023; Accepted: June 22, 2023.
Correspondence: Renata Rebello
Mendes, remendes@academico.ufs.br
How to cite
Mendes RR, Prado JRS. Unintentional doping:
A proposal for a risk reduction protocol based on an analytical review of the
risks and good practices of dietary supplementation in elite athletes. Rev Bras
Fisiol Exerc. 2023;22:e225468. doi: 10.33233/rbfex.v22i1.5468
Abstract
Introduction: Dietary
supplements have been considered the main cause of unintentional doping among
athletes; however, the prevalence of supplement use by elite athletes is almost
100%, and awareness of the risk of unintentional doping does not seem to be
effective. The literature has pointed out some possible factors related to the
poor quality of products available on the market; however, it does not propose
any protocol that objectively guides health professionals and athletes in terms
of reducing the risk of doping and damage to health caused by supplements
contaminated with prohibited substances not listed on the label. Objective:
To propose a protocol to reduce the risk of unintentional doping, based on a
narrative review of studies that have discussed regulatory factors related to
dietary supplements, prevalence of contamination of supplements, their main
contaminants, possible adverse effects on human health, awareness of athletes
about the indications for supplement use and the risks of unintentional doping.
Methods: Narrative review and document analysis on the websites of
international agencies related to anti-doping policy, such as the World
Anti-Doping Agency (WADA) and the Brazilian Authority for Doping Control
(ABCD). Results: A six-step protocol was developed, which proposes methods
potentially capable of reducing the risk of unintentional doping. Conclusion:
This instrument could be highly relevant in sports, especially among elite
athletes; however, without excluding the importance for any consumer of this
type of product, since high prevalence of contamination of food supplements and
consumption of this product were identified, as well as insufficient awareness
on the part of its consumers.
Keywords: dietary supplements; doping in
sports; athletic performance; security measures.
Resumo
Introdução: A suplementação alimentar tem sido
considerada a principal causa de doping não intencional entre atletas; no
entanto, a prevalência de consumo de suplementos por atletas de elite é próxima
a 100%, e a conscientização sobre o risco de doping não intencional não parece
ser efetiva. A literatura científica tem apontado alguns possíveis fatores
relacionados à má qualidade dos produtos disponíveis no mercado; entretanto,
não propõe qualquer protocolo que oriente, objetivamente, profissionais da área
da saúde e atletas quanto à redução de risco doping e danos à saúde causados
por suplementos contaminados com substâncias proibidas não listadas no rótulo. Objetivo:
Propor um protocolo de redução de risco de doping não intencional, a partir de
uma revisão narrativa sobre estudos que tenham discutido os fatores
regulatórios relacionados aos suplementos alimentares, prevalência de
contaminação de suplementos, seus principais contaminantes, possíveis efeitos
adversos à saúde humana, conscientização de atletas sobre as indicações para
uso de suplementos e os riscos de doping não intencional. Métodos:
Revisão narrativa e análise documental nos sites das agências internacionais
relacionadas à política antidopagem, como a Agência Mundial Antidopagem (WADA)
e a Autoridade Brasileira de Controle de Dopagem (ABCD). Resultados: Foi
elaborado um protocolo de seis passos, que propõe métodos potencialmente
capazes de reduzir o risco de doping não intencional. Conclusão: Esse
instrumento poderia ser de elevada relevância no âmbito esportivo,
especialmente entre atletas de elite, no entanto, sem excluir a importância
para outros consumidores desse tipo de produto, uma vez que foram identificadas
elevadas prevalências de contaminação de suplementos alimentares e de consumo desse
produto, bem como insuficiente grau de conscientização por parte de seus
consumidores.
Palavras-chave: suplementos nutricionais; doping nos
esportes; desempenho atlético; medidas de segurança.
Introduction
Elite athletes are constantly
submitted to challenges in which sports performance improvement stands out as a
central objective, either during training sessions or competitive events [1].
In this scenario, any evolution in performance, even if minimal, can be enough
to significantly influence an athlete’s result in a championship [2].
Among the factors capable of
promoting performance improvement in athletes, nutritional status stands out,
although some international positions mention that most of an athlete’s
nutritional needs can be achieved through simple food adjustments in their
routine [3]. Stellingwerff et al. [4] clarify
that some athletes may face difficulties making such adjustments viable.
In this sense, the two main types
of difficulties faced by athletes can be summarized as: a) limitations in the
consumption of adequate amounts of food, as athletes often mention not eating
enough due to gastrointestinal discomfort during training sessions or
competitions or because they experience reduced appetite and even changes in their
eating routine, due to constant international travel [4]; b) supply limitations
of specific ergogenic compounds, such as creatine, beta-alanine, caffeine,
nitrate, and sodium bicarbonate, considered safe and effective by the
International Olympic Committee (IOC), provided they are consumed in adequate
doses, which are not reached exclusively through food [5].
Faced with the difficulties of
meeting nutritional needs and specific ergogenic effects exclusively through
food, the of dietary supplements has been increasing among elite athletes,
ranging from 78 to 100% of this population, depending on the modality studied
[6,7]. However, it is essential to clarify that not all athletes face such
difficulties, which highlights the importance of individual nutritional
assessment [8].
For those athletes who need food
supplementation to achieve specific ergogenic effects and/or to maintain an
adequate nutritional status; and consequently prevent negative outcomes
resulting from malnutrition, such as overtraining syndrome and relative energy
deficiency in sport (REDS), it is necessary to clarify that this strategy is
considered the greatest risk factor for unintentional doping [9], being
responsible for approximately 6.4 and 8.9% of all adverse analytical findings
(positive doping) [10].
Doping has been defined as the
presence of prohibited substances and/or their metabolites in blood or urine
samples, and it is considered an unsportsmanlike crime, with the consequences
of losing titles, banning participation in competitions, compromising
reputation, and damage to health [11].
According to the principle of
“Strict Liability” provided for in Art. 14, paragraph III of the World
Anti-Doping Code [12], “it is not necessary that intent, fault, negligence or
knowing Use on the Athlete’s part be demonstrated in order to establish an
anti-doping violation”. In this way, when consuming a poor-quality food
supplement contaminated with substances prohibited by the World Anti-Doping
Agency (WADA) without any identification on the label, the athlete obtains
performance advantages in relation to his opponents, even if unintentionally.
Therefore, when presenting adverse analytical findings, they may suffer the
sanctions provided for this type of crime, as well as be exposed to the risk of
developing potential adverse health effects caused by prohibited substances.
The scientific literature has
pointed out some possible factors related to the poor quality of products
available on the market [11]. However, it does not propose any protocol that
objectively guides health professionals and athletes in reducing the risk of
doping and health damage caused by supplements contaminated with prohibited
substances not listed on the label.
Therefore, the first objective of
the present study was to carry out a narrative review of studies that have
discussed regulatory factors related to dietary supplements, the prevalence of
contamination of supplements, their main contaminants, possible adverse effects
on human health, awareness of athletes about the indications for supplements
use and the risks of unintentional doping. Based on this narrative review, the
second objective of the present study was to propose a protocol to reduce the
risk of unintentional doping.
Methods
The study consisted of a narrative
review and document analysis, in which conclusions culminated in a protocol
proposition to reduce the risk of unintentional doping.
The narrative review was carried
out through scientific articles indexed in the databases: PubMed, Google
Scholar, Science Direct and Web of Science, and Portal de Periódicos
CAPES, without language restrictions, through the terms “dietary supplements”,
“banned substances”, “contamination”, “cross-contamination”, “doping”,
“unintentional doping”, “involuntary doping”. Other relevant sources were found
in the references of related articles. No additional filters have been added,
and the last search was performed in February 2023.
For the document analysis, the
websites of governmental organizations and international agencies related to
the anti-doping policy were consulted, such as the World Anti-Doping Agency
(WADA) and the Brazilian Doping Control Authority (ABCD), and the last search
was carried out in February 2023.
Results and discussion
Regulatory factors related to dietary supplements
Unlike the rigid processes that
regulate the registration and marketing of a new drug, in much of the world,
the quality of food supplements is not tested before these products reach the
market [11].
In Europe and the United States
(USA), the producers themselves are responsible for “guaranteeing” the safety
of dietary supplements, with no need for proof before the product reaches the
market, which increases the risk of marketing poor-quality products [13].
In the US, the dietary supplement
industry is overseen by two federal agencies, the FDA and the Federal Trade
Commission (FTC), in which the first is in charge of the safety and proper labeling
of products, and the second of their advertising and promotional claims. In the
case of the FDA, its action is governed by the Dietary Supplement Health and
Education Act (DSHEA) of 1994, which classified these products as food and,
therefore, exempted them from proving their safety before being marketed [14].
In this country, drugs are considered unsafe until evidence shows otherwise;
food supplements are considered safe until proven they are not [15].
Thus, since manufacturers are not
required to submit safety information before marketing dietary supplements in
the US, the FDA depends on adverse event reports, product sampling, and
information from the scientific literature as evidence of risk. Consequently,
for an inappropriate product to be withdrawn from circulation in the US, it
must first have documented victims and be brought to the attention of health
authorities [15].
It is noteworthy that US supplement
manufacturers must comply with Good Manufacturing Practices (GMP) requirements,
which state that they "must establish - for each component and the
finished dietary supplement - specifications as to identity, purity, strength,
composition, and limits of contaminants, to assure its quality”. However, the
US GMP guidelines do not specify which tests and methods must be adopted, which
allows manufacturers to decide which methodologies they will base their quality
controls on. As a consequence, problems such as the presence of impurities,
microorganisms, toxins, and toxic elements such as lead, mercury, arsenic, and
cadmium in the products may occur, as well as the poor characterization and
replacement of declared components by cheaper alternatives, of lower quality
and even the inclusion, not informed on the label, of active ingredients prohibited
by WADA.
In Brazil, the regulation of the
dietary supplements sector is similar to the American and European ones, being
governed by the Resolution of the Collegiate Board (RDC) number 243 of July 26,
2018, of the National Health Regulatory Agency (Anvisa),
which provides for “the requirements for the composition, quality, safety, and
labeling of dietary supplements and for updating the lists of nutrients,
bioactive substances, enzymes, and probiotics, use limits, claims and
complementary labeling of these products” [16], plus the lists of permitted and
prohibited ingredients of Normative Instruction number 28 also of July 26,
2018, later modified by Normative Instruction number 76 of November 5, 2020.
As in the US, Brazil also regulates
dietary supplements more as food than as medicine, which translates into milder
and simpler standards and requirements for their registration and marketing,
and a lower degree of inspection. Unlike what happens in the USA; however,
Brazilian manufacturers must previously submit to Anvisa
information on the safety and efficacy of their products, which may include
scientific evidence such as clinical trials, endorsement by health authorities
or recognized regulatory bodies in other countries; in addition to of
pharmacopeias or other specific codes for the sector in Brazil or abroad [14].
In Brazil, until 2018, there was no
legal definition for dietary supplements. At that time, most of the products
used as dietary supplements were classified into different regulatory
categories: (I) Food for athletes; (II) Vitamin and/or mineral supplements;
(III) New foods and/or new ingredients; (IV) Foods with functional and/or
health properties; (V) Specific drugs; and (VI) Herbal Medicines. However, in
recent years, Anvisa has promoted a series of
debates, which led to RDC Anvisa nº 243/2018, which
defines the health requirements of dietary supplements and is characterized as
a regulatory framework in the country. Anvisa also established
food additives and technology adjuvants authorized for use in food supplements
through RDC Anvisa nº 239, of July 26, 2018 [17] and
published Normative Instruction nº 76, which provides for the updating of lists
of constituents, use limits, claims and complementary labeling of food
supplements; including minimum and maximum limits of nutrients, bioactive
substances, enzymes, and probiotics that may be contained in food supplements,
based on the daily recommendation for consumption of the product for certain
population groups indicated by the manufacturer [18].
RDC nº 243 [16] deals with
regulating the requirements for composition, quality, safety, and labeling of
food supplements, both for the industrial environment of large-scale production
and commercialization and for compounding pharmacies. Among several standards
established in this Resolution, the need to identify ingredients on the product
label stands out. More specifically, article 7 defines that “substances
considered as doping by the World Anti-Doping Agency are not allowed in the
composition of food supplements” (Table I). However, except for products
containing enzymes or probiotics, it is not mandatory to carry out analyzes of
food supplements before they are placed on the market, and the manufacturer is
solely responsible for declaring that he complies with the rules and
communicating the start of manufacture or importation of the product to the
local health surveillance agency. Added to this is the scarcity of official
methodologies in Brazil for carrying out this type of analysis, making the
quality and safety of these products questionable.
Table I - World anti-doping agency list
of prohibited substances and methods for the year 2023

Source: WADA - World Anti-Doping Agency
[19]
Cross-contamination is usually
attributed to errors in production in situations in which supplements and other
products containing substances prohibited by WADA are manufactured on the same
production line; in this case, containers or benches previously used in
handling prohibited substances are improperly cleaned and then reused for the
transport or storage of raw materials and food supplements, culminating in
contamination. There is also the hypothesis that the inclusion of the prohibited
substances in dietary supplements is intentional to increase the effectiveness
of the products and build consumer loyalty [20]. Additionally, the Brazilian
Doping Control Authority (ABCD) warns that, for the most part, contaminated
products are those often listed as possibly capable of reducing body weight and
promoting increased muscle anabolism [21], which corroborates with the
hypothesis of intentional contamination.
Therefore, as
the food supplements industry continues to grow and athletes continue to
consume them, the review and modernization of the regulation of this industry
are extremely necessary to prevent both acute and chronic damage to health, as
well as unintentional doping [11]. However, while the legislation is not
changed, the need to create mechanisms to reduce the risk of unintentional
doping is clear.
Prevalence of contamination of food supplements, their
main contaminants and possible adverse effects on human health
Passive exposure to prohibited
substances, caused by the consumption of dietary supplements, in addition to
causing damage to health, also makes elite athletes susceptible to
unintentional doping [22,23].
In a study by Kozhuharov,
Ivanov, and Ivanova [11], 50 studies were gathered and published between 1996
and 2021, in which 875 dietary supplements were analyzed as possible sources of
unintentional doping. The dietary supplements studied came from practically all
parts of the world, predominantly from the USA, Holland, United Kingdom, Italy,
and Germany, followed by products manufactured in China and Southeast Asia. The
authors found that of all the supplements analyzed, about 28% had a high risk
of unintentional doping due to the substances present but not declared on the
label.
Among these substances, the most
common was sibutramine in 248 contaminated food supplements (28.34%), followed
by testosterone and other anabolic steroids in 228 products (26.06%),
fluoxetine in 192 products (21.37%), 1,3-dimethylamylamine (DMAA) in 58
products (6.62%) and hygenamine in 15 (1.71%) of the
875 food supplements analyzed. Other substances such as diuretics and Selective
Androgen Receptor Modulators (SARMs) not declared on the labels and prohibited
by WADA were also identified in the products, but to a lesser extent (Table II)
[11].
Table II - Main contaminants found in
dietary supplements, and their frequency, not identified on the label
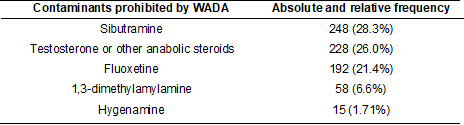
Source: Adapted from Kozhuharov, Ivanov, and Ivanova [11].
Food supplements, in general, are
used chronically, some even very frequently throughout the day. And if they are
contaminated with pharmacologically active substances, they can expose
consumers to serious side effects due to the accumulation of prohibited
substances without the slightest control of the ingested dose. Therefore, the
unexpected consumption of adulterated supplements can cause adverse effects
such as allergies, and cardiovascular, liver, and kidney problems, among
others, depending on the level of adulteration and the consumer's tolerance to
such substances [24].
According to Kozhuharov,
Ivanov, and Ivanova [11], the most recurrent substance in dietary supplements
as a contaminant was sibutramine. This substance poses serious health risks
both for elite athletes and for other individuals. Its ingestion can cause
several side effects, such as increased blood pressure, arrhythmias, dry mouth,
difficulty sleeping, headache, or joint and muscle pain [25,26,27].
Another type of substance that
stood out in the study by Kozhuharov, Ivanov, and
Ivanova [11] as a contaminant of food supplements was the category of anabolic
steroids; its adverse effects are correlated with prolonged use, which may lead
to interruption of the endogenous production of these hormones, as well as
infertility and gynecomastia. Additionally, they are associated with
cardiovascular side effects such as left ventricular hypertrophy, impaired
diastolic filling, hypertension, thrombosis, and hepatotoxicity [28,29,30].
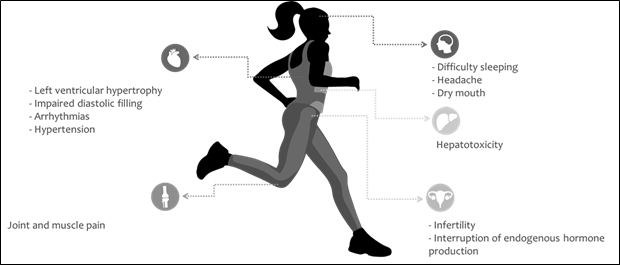
Source: Florentin et al. [25];
James et al. [26]; Scheen [27]; Torrisi et al. [28]; Karila et
al. [29]; Ivanova et al. [30]
Figure 1 - Major adverse health effects
caused by contaminants predominantly found in dietary supplements, such as
sibutramine, testosterone or other anabolic steroids, 1,3-Dimethylamylamine,
and hygenamine
As previously mentioned, the study
by Kozhuharov, Ivanov, and Ivanova [11] analyzed 50
studies published between 1996 and June 21, 2021, and found a prevalence of 28%
of contamination among food supplements. After this period, in our searches,
using the same descriptors and research platforms, we found four more studies
[31,32,33,34] whose objective was to evaluate the contamination of sports food
supplements.
Duiven et
al. [31] evaluated the prevalence of doping substances in a variety of
sports food supplements available in Dutch online stores. A total of 66 sports
supplements - identified by the study authors as "potentially high-risk
products" as their advertising claimed to modulate hormone regulation,
stimulate muscle mass gain, enhance fat loss, and/or increase energy - were
selected from 21 different brands and purchased in 17 virtual stores. All
products were analyzed for the presence of prohibited substances by a company
with extensive experience in anti-doping control. A total of 25 of the 66
products (38%) contained substances prohibited by the WADA, not declared on the
label, which included high levels of the stimulants oxylophrine,
β-methylphenethylamine and N,β-dimethylphenethylamine,
the stimulant 4-methylhexan-2-amine (methylhexaneamine,
1,3-dimethylamylamine, DMAA), the anabolic steroids boldenone
(1,4-androstadiene-3,17-dione) and 5-androstene-3β,17α-diol (17α-AED),
the beta-2 agonist higenamine and the beta-blocker
bisoprolol. The authors concluded that the ingestion of some products identified
in this study, in the concentrations found, could represent a significant risk
of unintentional doping violations and to consumers' health.
Leaney et
al. [32] carried out a study on the consumption of hygenamine
through products made from beetroot, currently evidenced as an ergogenic
strategy because it is a source of nitrate. To investigate this relationship,
concentrated beetroot beverages were consumed by six individuals, and this
compound was quantified in the urine. Hygenamine was
confirmed to be present in the majority of beet-derived foods and supplements
tested in this study, with experimental evidence that it can arise in beet
extracts upon heating. The results of this study demonstrate the first evidence
of a relationship between beetroot and hygenamine, a
substance prohibited by WADA.
It is noteworthy that, although
free hygenamine was detected in the urine of all
individuals tested in the study by Leaney et al.
[32], its concentration was significantly low, representing about 1% of the
acceptable limit described in the current WADA report. However, although the
risk of inadvertent doping violation by consuming the products investigated in
this study is low, beetroot as a source of hygenamine
should be considered by athletes, especially those who consume amounts higher
than those recommended by the manufacturers.
In the third study found in our
searches, after the publication of the systematic review by Kozhuharov,
Ivanov, and Ivanova [11], Zhang et al. [33] proposed a new analytical
method for the detection of anabolic steroids, also prohibited by WADA, in
samples of food supplements. The method was considered sensitive and accurate,
and when analyzing 300 liquid and solid food supplements, it detected a
positive sample for testosterone and three suspected drugs
(4-hydroxyandrostenedione, DHEA, and 6-Br androstenedione) in three food
supplements purchased on the internet.
Finally, in the last study found in
our searches, after the publication of the systematic review by Kozhuharov, Ivanov, and Ivanova [11], Rodriguez-Lopez et
al. [34] analyzed 52 “sports supplements” made of protein available in
physical and online stores in Spain with several objectives, among them, to
identify possible contamination with substances prohibited by the WADA. No
ingredients banned by WADA were found, except for colostrum in one of the
supplements, and consumption of colostrum is currently discouraged by WADA, as
it may contain growth factors (IGF-1), among others, which are prohibited and
can lead to doping.
When analyzing data from Zhang et
al. [33] and Rodriguez-Lopez et al. [34], it is possible to observe
a prevalence of contamination (1.33% and zero, respectively) lower than the 38%
reported by Duiven et al. [31] and the 28% mentioned
in the review by Kozhuharov, Ivanov, and Ivanova
[11]. However, in the first case, only one type of prohibited substance
(steroids) was evaluated, while in the second case, only protein supplements
marketed in a single country. While in the studies by Duiven
et al. [31] and Kozhuharov, Ivanov, and
Ivanova [11], there was an investigation of numerous types of prohibited
substances, specifically, in the case of Kozhuharov,
Ivanov, and Ivanova [11], it is a systematic review with meta-analysis, which
brought together supplements from all over the world, which reinforces the need
for extreme vigilance concerning the consumption of food supplements by
athletes.
Athletes' awareness of dietary supplements and the
risk of unintentional doping
The most cited motivations for
dietary supplements use by athletes around the world have been improvements in
performance, health, and recovery. Additionally, women are more likely to
consume supplements for health reasons, while men more often report using them
to enhance sports performance [6,35]. However, according to Walpurgis et al.
[20], most athletes using dietary supplements are not aware of the consequences
of consuming contaminated dietary supplements, such as unpredictable health
risks and adverse analytical discovery in routine doping controls.
Studies have found that athletes'
main sources of information on the subject tend to be of low quality, as they
mostly come from their coaches, team partners, friends, or even family members
[35,36,37]. According to Dodge [38], consumers of dietary supplements mistakenly
believe that if dietary supplements are approved by the government, then they
are tested for safety and efficacy, as well as have their content analyzed in
the laboratory, and manufacturers are obliged to disclose adverse effects on
consumers. However, this is not observed in practice since the laws of several
countries do not require such procedures.
A study by Braun et al. [39]
found that only 36% of the participating athletes knew that food supplements
could have some type of contaminant. Following by Torres-McGehee et al.
[40], in which only 9% of 400 North American athletes had adequate knowledge
about sports nutrition, including supplementation. Another study with college
athletes showed that 86% were unaware that food supplements could have
potential adverse effects [37]. In addition to these, a study carried out with
Australian athletes on the same subject showed that 62% of respondents did not
know the active ingredient(s) of the supplement(s) they consumed, 57% did not
know about the possible adverse effects, 54% were unaware of the mechanism of
action, and 52% were unaware of the recommended dose [41].
Chan et al. [42] evaluated
410 young athletes (17.7 years old ± 3.9), Australian, from regional, national,
and international levels, from modalities such as Athletics, Badminton,
Swimming, Gymnastics, Swimming, Triathlon, Basketball, Cricket, Football,
Rugby, hockey and water polo. Such athletes received a lollipop free of charge
while waiting for the completion of a questionnaire. Among the various findings
of the study, it was observed that only 40.6% refused to eat an unknown food
given to them and that among all those who consumed the product, only 16.1%
read the list of ingredients before making it. This study suggests that young
athletes had a low level of concern about exposing themselves to new food
products and the possible risks of unintentional doping.
Some countries, such as Germany,
France, the United Kingdom, Austria, and Holland, have databases available for
athletes, which catalog dietary supplements tested for ingredients [20].
Specifically, in Holland in 2003, the Dutch Safeguards System for Dietary
Supplements in Elite Sport, known as NZVT, was created [43]. Thus, Wardenaar et al. [44] tested the knowledge and
attitudes of 601 Dutch athletes with Olympic and non-Olympic status towards the
NZVT system. The authors showed that, although the majority (68%) of athletes
were aware that dietary supplements can lead to an adverse analytical finding,
and 87.8% of these athletes considered fraud due to incomplete labeling
unacceptable, there is still a reasonable portion of these athletes (32%) who
are unaware of such risks. Of the athletes who were aware of the NZVT system,
those with Olympic status reported using it more frequently than non-Olympic
athletes (81.7% vs. 50.0%, p < 0.001). Additionally, women were more
familiar with and used the system more frequently when compared to men. In
conclusion, the authors report that although doping warnings and regulations
have been in place, considering the risk of unintentional use of doping for
more than two decades, knowledge of the Olympic and non-Olympic status of
high-level athletes still needs to be improved.
The studies cited above show that
even athletes from countries with programs or systems that guide the purchase
of safe products are still insufficiently aware of the risk of unintentional
doping caused by the consumption of contaminated food supplements. According to
our searches, there is no robust investigation on the subject in Brazil, but
considering that in this country there is no governmental system to protect
athletes in this sense, it is believed that the level of awareness is even lower,
which raises the question of the need for educational programs in this regard
and the proposition of risk reduction protocols.
Proposal for a Protocol to reduce the risk of
unintentional doping through dietary supplements
Considering that the risk of
unaware athletes consuming dietary supplements contaminated with substances
prohibited by WADA is high, but also considering that the use of these products
may be indispensable in certain elite sports scenarios and that changes in
regulatory factors are unlikely to occur in the short term, the proposal of a
risk reduction protocol becomes fundamental. In this way, our proposal will be
exposed in 6 steps:
1st Step: Consume only food supplements
that are strictly necessary and that present scientific evidence of efficacy
and safety.
Few commercially available products
claiming ergogenic benefits are supported by solid evidence. Research
methodologies on the effectiveness of sports supplements are often limited by
small sample sizes, the inclusion of untrained individuals, low representation
of specific subpopulations of athletes (women, older athletes, athletes with
disabilities, etc.), performance tests that are unreliable or irrelevant, poor
control of confounding variables, by not including control of the athletes'
diet during the study or by not considering the interaction with other
supplements [45,46].
The International Olympic
Committee, in a position released recently [5], categorized food supplements
according to the purpose of use and the degree of evidence regarding safety and
efficacy. Following these criteria, the following categories emerged:
supplements whose purposes are: (a) Practically providing energy and nutrients,
(b) Preventing and/or treating nutritional deficiencies, (c) Promoting muscle
mass gain, (d) Promoting weight loss, (e) Promote performance improvement
indirectly, through injury prevention and immunity improvement, (f) Promote
performance improvement directly.
According to Table III, in the
category “supplements whose purpose is to directly promote performance
improvement”, for example, there is evidence that only five dietary supplements
- creatine, sodium bicarbonate, beta-alanine, caffeine, and nitrate - could
promote gains in performance margins, as long as they are used in specific
scenarios [5]. Thus, in practice, the athlete should stick to just such
possibilities, also considering the guidance of a nutritionist to assess the
feasibility of use according to the specific scenario, such as training
periodization, the specificity of each sport modality, and the biological
individuality of the athlete.
Table III - Categorization of food
supplements according to purpose of use and degree of evidence regarding safety
and efficacy
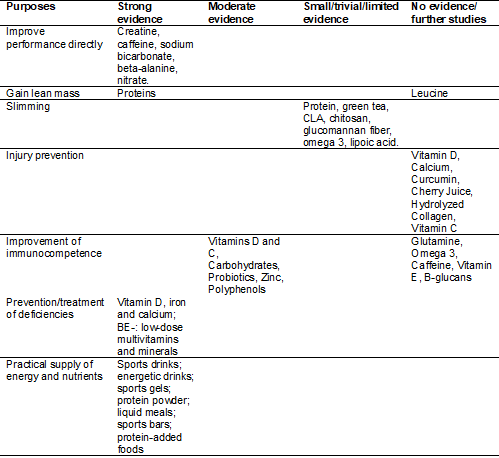
Source: Adapted from Maughan et
al. [5]
In addition to the positions
published by specific entities such as IOC [5], ISSN [3], and ACSM [47], the
nutritionist responsible for prescribing dietary supplements can use other
types of studies for decision-making. In this case, the professional must
define his conduct based on scientific evidence, as suggested in Figure 2, understanding
that the highest level of reliability has been found in systematic review
studies with or without meta-analysis.
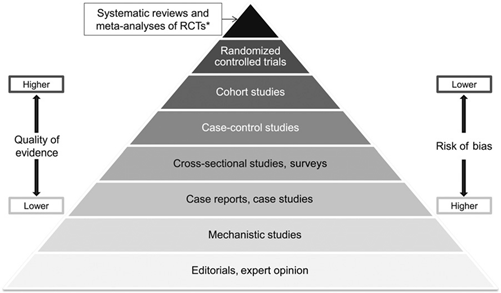
*RCTs = Randomized clinical trials.
Source: Pereira and Veiga [48]
Figure 2 - Hierarchy of evidence used to
establish good health practices
Finally, if there is still no
systematic review with meta-analysis available for a given dietary supplement
and for the desired population, clinical trials can be useful in
decision-making, as long as they are interpreted critically, considering
criteria such as sample type, type of performance testing, study design,
supplement quality, study funding, and conflict of interest, among others, as
discussed by Porrini and Del Bo [49].
Thus, the nutritionist responsible
for prescribing dietary supplements must be able to interpret such studies,
base their conduct on scientific evidence, and guide athletes to use only what
is strictly necessary.
2nd step: Analyze the quality of the
product available on the market
Evaluate ingredient list
The first type of analysis refers
to something that precedes unintentional doping; that is, at first, the athlete
must be aware of the fact that it is his responsibility to analyze all the
compounds present in the list of ingredients, even though the marketing is more
directed to only one ingredient. In this case, if the prohibited substance is
described in the list of ingredients, it will no longer be characterized as
unintentional doping.
Analyze in the laboratory the substances present in
the food supplement, regardless of not being present in the list of ingredients
Considering that about 28% to 38%
of the products available on the market contain substances prohibited by WADA,
without these being described in the list of ingredients, step 2.1. is
essential, but not enough. To ensure that the product is free of contaminants,
it would be necessary to send a sample of it for laboratory analysis [11], and
if the result points to the presence of a contaminant, the athlete should
discard it and look for another option on the market.
However, such procedures are often
significantly costly for the athlete. Analyzing each product reduces the speed
of the process and can represent an unfeasible cost for most athletes. Thus, to
reduce such obstacles, there are two distinct initiatives, but still not very
effective in global terms: a) companies that sell food supplements tested in
batches (batch-tested) and certify such products, including seals on the
packaging, so that the consumer can identify it. However, they are rare on the
market and therefore difficult to access; b) Government programs aimed at
analyzing supplements and disclosing to athletes a list of tested and approved
products and their respective batches. However, according to our searches so
far, only five countries have this type of program: Germany, the United
Kingdom, France, Holland, and Austria [11].
It is important to point out that
each new batch of food supplement produced by the same company must be tested
again since it was probably handled at different times and perhaps under
different circumstances.
Investigate the history of the manufacturing company
In the impossibility of performing
analysis of a dietary supplement in the laboratory, either on the athlete's
initiative, by laboratories that perform analysis and disclose the seal on the
product label, or by governmental initiatives, Kozhuharov,
Ivanov, and Ivanova [11] suggest that, if the use of the product is crucial for
the health and performance of the athlete, before acquiring it, the athlete must
investigate the history of the manufacturing company, in search of possible
previous accusations. In this same line of reasoning, it is common for elite
athletes from the same team to share experiences, the most experienced ones report to the most novices the brands that they have
been using for years and never generated adverse analytical findings in the
constant anti-doping controls to which they are submitted. The fact that a
company has never been denounced or caught in cases of contamination does not
prevent it from committing possible fraud in the future; however, according to Kozhuharov, Ivanov, and Ivanova [11], it is a reasoning of
reduction of probabilities. And for that reason, in parallel with this strategy
(step 2.3 of our protocol), more preventive measures should be taken, as
described in the following steps.
3rd step: Acquisition of dietary
supplements preferably produced in large-scale industry
The risks of contamination in
dietary supplements with prohibited substances are real; both in products
produced in industry, on a large scale, and in those manipulated in pharmacies.
However, according to ABCD [21], there is the possibility that part of the
compounding pharmacies does not strictly and consistently follow the criteria
required of manufacturers of industrialized products sold on a large scale, and
for this reason, they would represent a greater risk for consumers.
Additionally, Judkins, Teale, and Hall [50] suggest that some pharmacies that
handle medicines and dietary supplements may use contaminated or low-quality
raw materials, and work with unreliable substances, creating unsafe mixtures
with ingredients that have not yet been tested in humans [21].
However, our searches found that
scientific studies have been dedicated to assessing the presence of prohibited
substances in supplements produced on a large scale without necessarily
comparing their contamination rates with products handled in pharmacies
[51,52,53,54,55,56]. Therefore, given this scientific gap, it is not possible to state that
supplements produced on a large scale are less susceptible to contamination
than those manipulated in pharmacies.
While this gap is not filled
through new scientific evidence, in compliance with the ABCD and the document
still in force [21], our unintentional doping risk reduction protocol will
adopt the proviso that the preferential acquisition must be of products
produced in industry, without excluding, however, the option of products
manipulated in pharmacy. Additionally, if the athlete decides to purchase a
product compounded in a pharmacy, it is recommended to verify that the chosen
pharmacy follows the rules of good practices for handling magistral and
officinal preparations for human use in pharmacies.
4th step: Storage of possible evidence of
“unintentional” doping by the athlete (WADA, 2021)
As previously mentioned, the
unintentional doping of the athlete does not exempt them from possible punitive
consequences because even if unconsciously, this subject obtained advantages
over their opponents. However, it has been observed that in cases that athletes
claim and prove the absence of intent, sanctions can be mitigated. It is also
essential that possible contamination of food supplements can be identified, so
that measures can be taken in relation to their manufacturers. Therefore, it is
suggested that the following items be stored.
Duplicate of the same batch number of the food
supplement
Given step 1, that is, defining the
food supplement(s) essential for the health and/or performance of the elite
athlete, and step 2.3, the investigation of the history of the product
manufacturer desired (since it is impossible to analyze each product in the
laboratory), the next preventive measure consists of purchasing the product
together with a duplicate of the same lot number (step 3).
Since the early 2000s, it has been
accepted by the Court of Arbitration for Sport in Lausanne (Switzerland) that,
in some specific circumstances, unusual explanations can be provided to the
Panel to explain an adverse analytical finding (positive doping). This change
was considered the “opening of doors” for forensic investigations, as is done
in criminal courts. Therefore, a forensic approach may include testing
prohibited substances in food and beverages, but especially in dietary
supplements [57].
According to WADA [2021], the
athlete must keep a sample of the food supplement stored in a safe place,
preferably a duplicate with the same batch number and sealed. Thus, if the
athlete presents adverse analytical findings (positive doping), such duplicate
may be submitted for analysis to detect possible contamination with the
substance detected in the sample collected from the athlete. Therefore, this
duplicate should not be consumed (Figure 3).
In practice, the sealed duplicate
storage orientation may represent a substantial increase in the athletes'
monthly budget, as it means doubling the budget allocated to food
supplementation. However, when the use of a food supplement is common
throughout the season, such as a certain type of carbohydrate for intra-workout
consumption, it is possible to predict the amount to be consumed for a longer
period (for example, six months) and organize the acquisition of a greater
number of packs, all from the same lot, keeping only one duplicate for the six
months, which would have less impact on the athlete's budget. In this example,
it is essential to observe the product's shelf life.

Figure 3 - Food supplement storage
suggestion: for each product to be consumed, a duplicate with the same batch
number must be stored
Invoice in the name of the athlete, with product
description and batch number
In addition, to store a duplicate
of the food supplement consumed by an athlete, it is essential to prove that
the product was purchased by them. Therefore, the ABCD [21] guides the athlete
to store a copy of the invoice, which describes the name and ID number of the
athlete and the batch number of the purchased products. If the invoice is
generated automatically by some computerized system of the commercial
establishment and does not describe the lot number of the products, it is worth
requesting a separate statement listing the name and ID number of the athlete
and the lot numbers of the products purchased by them.
In practice, the athlete may
receive food supplementation from a sponsor, and the invoice would not be a
document involved in the process. In this case, it would be interesting for the
athlete to ask the sponsor for a duplicate of the food supplement with the same
batch number, accompanied by a signed and dated declaration, certifying that
the product, with that batch number, is being donated to the athlete, with the
name and ID number identified.
Nutritionist's prescription, with their license
number, professional stamp and signature
Under the World Anti-Doping Code,
which outlines the principle of strict liability, athletes are liable even when
a doping compound enters their bodies without their knowledge. It is the
personal duty of athletes to ensure that non-permitted substances do not enter
their bodies, and therefore, when instructed to consume a dietary supplement,
they must be aware that they are assuming the risk of unintentional doping.
However, if an
athlete is caught with positive doping and claims that the fact is due to
supposedly contaminated food supplements, ABCD [21] considers that proof of
prescription by a professional can strengthen the athlete's defense in court
responsible for this type of process.
5th step: Acquisition in physical stores
According to ABCD [21] guidelines,
online sales facilitate the sale and distribution of not safe and/or legal
products since, in most cases, sellers can close the company or change the name
or the page of the internet from another country. Additionally, the anonymity
and ease of opening and closing an online business has made the illegal
distribution of steroids, which can contaminate dietary supplements, a serious
problem.
On the other hand, in our searches,
no studies were found that compared different ways of acquiring food
supplements contaminated with substances prohibited by WADA, which suggests
that the hypothesis that “products sold online pose a greater risk of
contamination than those sold physically” has yet to be scientifically tested.
However, in
practice, the dietary supplements acquisition in duplicate, with the same lot
number (according to step 4 of the proposed protocol), is more feasible in
physical stores, as it is not always possible to choose the lot numbers when
making an online purchase.
6th step: Adequate completion of the
specific form when collecting a urine or blood sample for anti-doping analysis
When an athlete undergoes
anti-doping tests, in addition to collecting a urine or blood sample for later
laboratory analysis, it is also necessary to fill out a form through which some
facts are questioned, including the food supplements usage. In this way, it is
fundamental that the athlete registers all the food supplements that they are
consuming or that they recently have; because if they forget to mention the use
of these compounds and are caught with a positive test for prohibited
substances, it would be incoherent to claim that the cause of the contamination
is the food supplement.
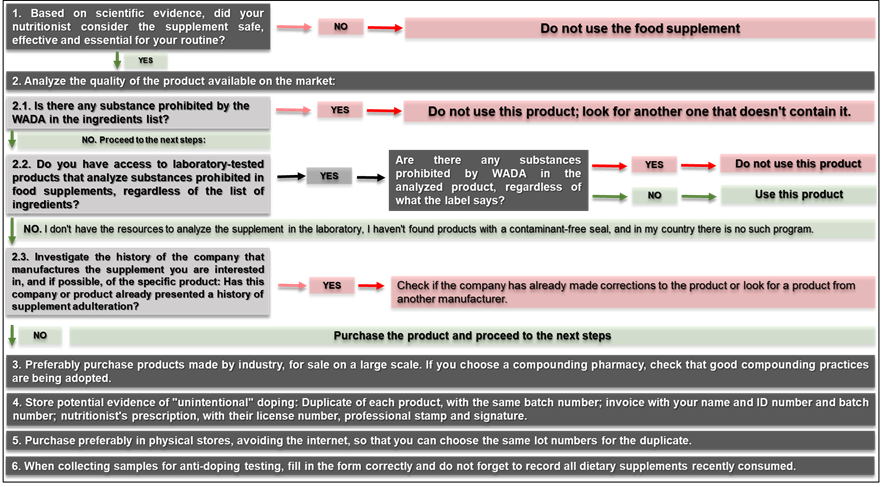
Figure 4 - Unintentional doping risk
reduction protocol
Conclusion
The use of contaminated food
supplements is an important predictor of doping, and regulatory factors play a
fundamental role in the high availability of poor-quality products on the
market. The prevalence of contamination of supplements varies between 28 and
38%, with emphasis on sibutramine, testosterone, and other anabolic steroids,
fluoxetine, 1,3-dimethylamylamine, and hygenamine,
which, in addition to causing doping, are also capable of causing harm to the
health of the consumer. The degree of awareness of athletes about the subject
is low, and for this reason, we proposed a risk reduction protocol consisting
of six steps:
(1) Seek only strictly necessary
food supplements that present scientific evidence of efficacy and safety,
guided by a nutritionist;
(2) Analyze the quality of the
product available on the market: read the list of ingredients and arrange for
the product to be analyzed in the laboratory, either on your initiative or by
companies that test in batches and issue a quality seal, or through programs
government, if your country has such a strategy; if laboratory analysis is not
feasible, investigate the manufacturer's history, choose companies without
cases of adulteration, and move on to the next steps;
(3) Preferably purchase products
made by industry, or if you choose a compounding pharmacy, check that good
practices are being adopted for handling magistral and officinal preparations
for human use in pharmacies;
(4) Store possible evidence of
“unintentional” doping: duplicate product with the same batch number; invoice
with the athlete's name and product description with the batch number; the
nutritionist prescription, with their license number, professional stamp, and
signature;
(5) Acquire supplements preferably
in physical stores, avoiding the internet, so that you can choose the same
batch numbers;
(6) At the time of collecting
samples for anti-doping testing, fill in the form correctly and do not forget
to record all food supplements consumed recently.
Conflict of interest
There are no conflicts of interest.
Financing source
There were no external sources of
funding for this study.
Authors' contribution
Research conception and
design: Mendes RR; Data collection: Mendes RR, Prado
JRS; Data
analysis and interpretation: Mendes RR, Prado JRS;
Statistical analysis: Mendes RR; Manuscript writing: Mendes RR, Prado
JRS; Critical review of the manuscript for important intellectual content:
Mendes RR
References
- Maughan RJ, Shirreffs SM, Vernec A. Making decisions about supplement use. Int J
Sport Nutr Exerc Metab. 2018;28(2):212-9. doi: 10.1123/ijsnem.2018-0009 [Crossref]
- Guest NS, Van Dusseldorp TA,
Nelson MT, Grgic J, Schoenfeld BJ, Jenkins NDM, et
al. International society of sports nutrition position stand: caffeine and
exercise performance. J Int Soc Sports Nutr.
2021;18(1):1. doi: 10.1186/s12970-020-00383-4 [Crossref]
- Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. 2018;15(1):38. doi: 10.1186/s12970-018-0242-y [Crossref]
- Stellingwerff T, Heikura IA, Meeusen R, Bermon S, Seiler S, Mountjoy ML, et al. Overtraining Syndrome (OTS) and Relative Energy Deficiency in Sport (RED-S): shared pathways, symptoms and complexities. Sports Med. 2021;51(11):2251-80. doi: 10.1007/s40279-021-01491-0 [Crossref]
- Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE,
Peeling P, Phillips SM, et al. IOC consensus statement: dietary supplements and
the high-performance athlete. Br J Sports Med. 2018;52(7):439-55. doi: 10.1136/bjsports-2018-099027 [Crossref]
- Daher J, Mallick M, El Khoury D. Prevalence of dietary supplement use among athletes worldwide: a scoping review. Nutrients. 2022;14(19). doi: 10.3390/nu14194109 [Crossref]
- Lauritzen F, Gjelstad A. Trends in dietary supplement use among athletes selected for doping controls. Front Nutr. 2023;10:1143187. doi: 10.3389/fnut.2023.1143187 [Crossref]
- Thomas M, Kalicinski M. The effects of slackline balance training on postural control in older adults. J Aging Phys Act.2016;393-8. doi: 10.1123/japa.2015-0099 [Crossref]
- Hurst P. Are dietary supplements a gateway to doping? A retrospective survey of athletes’ substance use. Subst Use Misuse. 2023;58(3):365-70. doi: 10.1080/10826084.2022.2161320 [Crossref]
- Outram S, Stewart B. Doping through supplement use: a review of the available empirical data. Int J Sport Nutr Exerc Metab. 2015;25(1):54-9. doi: 10.1123/ijsnem.2013-0174 [Crossref]
- Kozhuharov VR, Ivanov K, Ivanova S. Dietary supplements as source of unintentional doping. Biomed Res Int. 2022;2022:8387271. doi: 10.1155/2022/8387271 [Crossref]
- WADA - World Anti-Doping Agency. Anti-doping code.
[Internet] 2021 [cited 2023 Jul 24]. Available from:
https://www.wada-ama.org/en/resources/world-anti-doping-program/world-anti-doping-code.
- Wierzejska RE. Dietary supplements-for whom? The current state of knowledge about the health effects of selected supplement use. Int J Environ Res Public Health. 2021;18(17). doi: 10.3390/ijerph18178897 [Crossref]
- Richardson E, Akkas F,
Cadwallader AB. What should dietary supplement oversight look like in the US?
AMA J Ethics. 2022;24(5):E402-9. doi: 10.1001/amajethics.2022.402 [Crossref]
- Cadwallader AB. Which features of dietary supplement industry, product trends, and regulation deserve physicians’ attention? AMA J Ethics. 2022;24(5):E410-18. doi: 10.1001/amajethics.2022.410 [Crossref]
- Brasil.
Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA).
Resolução da Diretoria Colegiada – RDC n°243, de 26 de julho de 2018. Dispõe
sobre os requisitos sanitários dos suplementos alimentares. Diário Oficial da
União. Brasília: Diário Oficial da União; 2018.
- Molin TRD, Leal GC, Muratt
DT, Marcon GZ, Carvalho LM, Viana C. Regulatory framework for dietary
supplements and the public health challenge. Rev Saúde Pública. 2019;53:90. doi: 10.11606/s1518-8787.2019053001263 [Crossref]
- Brasil.
Instrução Normativa - IN No 76, de 5 de novembro de 2020. Dispõe sobre a
atualização das listas de constituintes, de limites de uso, de alegações e de
rotulagem complementar dos suplementos alimentares. Brasília: Diário Oficial da
União; 2020.
- WADA - World Anti-Doping Agency. The prohibited list. [Internet]. 2023. [cited 2023 Jul 24]. Available from: https://www.wada-ama.org/en/prohibited-list
- Walpurgis K, Thomas A, Geyer H, Mareck U, Thevis M. Dietary supplement and food contaminations and their implications for doping controls. Foods. 2020;9(8). doi: 10.3390/foods9081012 [Crossref]
- ABCD -
Autoridade Brasileira de Controle de Dopagem. Apostila Antidopagem. {Internet].
2022. [cited 2023 Jul 24]. Available
from: https://www.gov.br/abcd/pt-br/composicao/educacao-e-prevencao/material-educativo-antidopagem-1.
2022
- Anderson JM. Evaluating the athlete’s claim of an
unintentional positive urine drug test. Curr Sports Med
Rep.2011;10(4):191-6. doi: 10.1249/JSR.0b013e318224575f [Crossref]
- Silva LFM, Ferreira KS. Segurança alimentar de suplementos comercializados no Brasil. Rev Bras Med Esporte. 2014;20(5). doi: 10.1590/1517-86922014200501810 [Crossref]
- Henao MMM. Desenvolvimento e aplicação de
método analítico para detecção de estimulantes em suplementos nutricionais
adulterados. São Paulo: Universidade de São Paulo; 2018.
- Florentin M, Liberopoulos EN, Elisaf MS. Sibutramine-associated adverse effects: a practical guide for its safe use. Obes Rev. 2008;9(4):378-87. doi: 10.1111/j.1467-789X.2007.00425.x [Crossref]
- James WPT, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905-17. doi: 10.1056/NEJMoa1003114 [Crossref]
- Scheen AJ. Cardiovascular risk-benefit profile of sibutramine. Am J Cardiovasc Drugs. 2010;10(5):321-34. doi: 10.2165/11584800-000000000-00000 [Crossref]
- Torrisi M, Pennisi G, Russo I, Amico F, Esposito M, Liberto A, et al. Sudden cardiac death in anabolic-androgenic steroid users: a literature review. Medicina (Kaunas). 2020;56(11). doi: 10.3390/medicina56110587 [Crossref]
- Karila T, Hovatta O, Seppälä T. Concomitant abuse of anabolic androgenic steroids and human chorionic gonadotrophin impairs spermatogenesis in power athletes. Int J Sports Med. 2004;25(4):257-63. doi: 10.1055/s-2004-819936 [Crossref]
- Ivanova S, Ivanov K, Pankova
S, Peikova L. Consequences of anabolic steroids
abuse. Farmatsiia [Internet]. 2014 [cited 2022 Set
12];61(4):44-50. Available from:
https://www.researchgate.net/publication/287323821_Consequences_of_anabolic_steroids_abuse
- Duiven E, van Loon LJC, Spruijt L, Koert W, de Hon OM. Undeclared doping substances are highly prevalent in commercial sports nutrition supplements. J Sports Sci Med. 2021;20(2):328-38. doi: 10.52082/jssm.2021.328 [Crossref]
- Leaney AE,
Heath J, Midforth E, Beck P, Brown P, Mawson DH.
Presence of higenamine in beetroot containing
“foodstuffs” and the implication for WADA-relevant anti-doping testing. Drug Test
Anal. 2023;15(2):173-80. doi: 10.1002/dta.3383 [Crossref]
- Zhang Y, Wu X, Wang W, Huo J, Luo J, Xu Y, et al. Simultaneous detection of 93 anabolic androgenic steroids in dietary supplements using gas chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2022;211:114619. doi: 10.1016/j.jpba.2022.114619 [Crossref]
- Rodriguez-Lopez P, Rueda-Robles A, Sánchez-Rodríguez L, Blanca-Herrera RM, Quirantes-Piné RM, Borrás-Linares I, et al. Analysis and screening of commercialized protein supplements for sports practice. Foods. 2022;11(21). doi: 10.3390/foods11213500 [Crossref]
- Mallick M, Camacho CB, Daher J, El Khoury D. Dietary supplements: a gateway to doping? Nutrients. 2023;15(4):881. doi: 10.1080/10826084.2022.2161320 [Crossref]
- Froiland K, Koszewski W, Hingst J, Kopecky L. Nutritional supplement use among college athletes and their sources of information. Int J Sport Nutr Exerc Metab. 2004;14(1):104-20. doi: 10.1123/ijsnem.14.1.104 [Crossref]
- Tian HH, Ong WS, Tan CL. Nutritional supplement use
among university athletes in Singapore. Singapore Med J. 2009;50(2):165-72.
https://pubmed.ncbi.nlm.nih.gov/19296032/
- Dodge T. Consumers’ perceptions of the dietary supplement health and education act: implications and recommendations. Drug Test Anal. 2016;8(3-4):407-9. doi: 10.1002/dta.1857 [Crossref]
- Braun H, Koehler K, Geyer H, Kleiner J, Mester J, Schanzer W. Dietary supplement use among elite young German athletes. Int J Sport Nutr Exerc Metab. 2009;19(1):97-109. doi: 10.1123/ijsnem.19.1.97 [Crossref]
- Torres-McGehee TM, Pritchett KL, Zippel D, Minton DM, Cellamare A, Sibilia M. Sports nutrition knowledge among collegiate athletes, coaches, athletic trainers, and strength and conditioning specialists. J Athl Train. 2012;47(2):205-11. doi: 10.4085/1062-6050-47.2.205 [Crossref]
- Baylis A, Cameron-Smith D, Burke LM. Inadvertent doping through supplement use by athletes: assessment and management of the risk in Australia. Int J Sport Nutr Exerc Metab. 2001;11(3):365-83. doi: 10.1123/ijsnem.11.3.365 [Crossref]
- Chan DKC, Donovan RJ, Lentillon-Kaestner V, Hardcastle SJ, Dimmock JA, Keatley DA, et al. Young athletes’ awareness and monitoring of anti-doping in daily life: Does motivation matter? Scand J Med Sci Sports. 2015;25(6):e655-63. doi: 10.1111/sms.12362 [Crossref]
- Hon O, Coumans B. The continuing story of nutritional supplements and doping infractions. Br J Sports Med. 2007;41(11):800-5; discussion 805. doi: 10.1136/bjsm.2007.037226 [Crossref]
- Wardenaar FC, Hoogervorst D, Vento KA, de Hon PhD O. Dutch olympic and non-olympic athletes differ in knowledge of and attitudes toward third-party supplement testing. J Diet Suppl. 2021;18(6):646-54. doi: 10.1080/19390211.2020.1829248 [Crossref]
- Erdman J, Oria M, Pillsbury L. Nutrition and traumatic brain injury: improving acute and subacute health outcomes in military personnel. In: Erdman J, Oria M, Pillsbury L, eds. Washington, DC: National Academies Press; 2011. doi: doi: 10.17226/13121 [Crossref]
- Lewis M, Ghassemi P, Hibbeln J. Therapeutic use of omega-3 fatty acids in severe head trauma. Am J Emerg Med. 2013;31(1):273e5-8. doi: 10.1016/j.ajem.2012.05.014 [Crossref]
- Thomas DT, Erdman KA, Burke LM. American College of
Sports Medicine joint position statement. Nutrition and athletic performance.
Med Sci Sports Exerc. 2016;48(3):543-68. doi: 10.1249/MSS.0000000000000852 [Crossref]
- Pereira
C, Veiga N. Educação para a saúde baseada em evidências. Millenium. Journal of Education Technologies and Health
[Internet]. 2014 [cited 2022 Jun 12];46(46):107-36. Available from:
https://revistas.rcaap.pt/millenium/article/view/8144
- Porrini M, Del Bo? C. Ergogenic aids and supplements. Front Horm Res. 2016;47:128-52. doi: 10.1159/000445176 [Crossref]
- Judkins CMG, Teale P, Hall DJ. The role of banned substance residue analysis in the control of dietary supplement contamination. Drug Test Anal. 2010;2(9):417-20. doi: 10.1002/dta.149 [Crossref]
- Kamber M, Baume N, Saugy M, Rivier L. Nutritional supplements as a source for positive doping cases? Int J Sport Nutr Exerc Metab. 2001;11(2):258-63. doi: 10.1123/ijsnem.11.2.258 [Crossref]
- Khazan M, Hedayati M, Kobarfard F, Askari S, Azizi F. Identification and
determination of synthetic pharmaceuticals as adulterants in eight common
herbal weight loss supplements. Iran Red Crescent Med J. 2014;16(3):e15344. doi: 10.5812/ircmj.15344 [Crossref]
- Mathews NM. Prohibited contaminants in dietary
supplements. Sports Health.
2018;10(1):19-30. doi: 10.1177/1941738117727736 [Crossref]
- Gueorguieva EP, Ivanov K, Gueorguiev S, Mihaylova A, Madzharov V, Ivanova S. Detection of sibutramine in herbal food supplements by UHPLC/HRMS and UHPLC/MS-MS. Biomedical Research. 2018;29(14). doi: 10.4066/biomedicalresearch.29-18-879 [Crossref]
- Cohen PA, Travis JC, Keizers PHJ, Boyer FE, Venhuis BJ. The stimulant higenamine in weight loss and sports supplements. Clin Toxicol (Phila). 2019;57(2):125-30. doi: 10.1080/15563650.2018.1497171 [Crossref]
- Leaney AE, Beck P, Biddle S, Brown P, Grace PB, Hudson SC, et al. Analysis of supplements available to UK consumers purporting to contain selective androgen receptor modulators. Drug Test Anal. 2021;13(1):122-7. doi: 10.1002/dta.2908 [Crossref]
- Kintz P. The forensic response after an adverse analytical finding (doping) involving a selective androgen receptor modulator (SARM) in human athlete. J Pharm Biomed Anal. 2022;207:114433. doi: 10.1016/j.jpba.2021.114433 [Crossref]
