Rev Bras
Fisiol Exerc. 2023;22:e225470
ORIGINAL ARTICLE
Reproducibility of inhibitory control, working memory,
and cognitive flexibility measures in older women
Reprodutibilidade
de medidas do controle inibitório, memória de trabalho e flexibilidade
cognitiva em mulheres idosas
Alan
Pantoja-Cardoso1, José Carlos Aragão-Santos1, Marcos
Raphael Pereira Monteiro1, Poliana de Jesus Santos1, Ana
Carolina Dos-Santos1, Heloiana Faro2, Juan Ramon Heredia-Elvar3,
Leonardo de Sousa Fortes2, Marzo Edir Da Silva-Grigoletto1
1Universidade
Federal de Sergipe, São Cristóvão, SE, Brasil
2Universidade
Federal de Paraíba, Brasil
3Universidad
Alfonso X El Sabio, Madrid, Espanha
Received: February 2, 2023; Accepted: March 5, 2023.
Correspondence: Alan Pantoja Cardoso, alan_pantoja1996@hotmail.com
Como citar
Pantoja-Cardoso A, Aragão-Santos JC, Monteiro MRP, Santos PJ,
Dos-Santos AC, Faro H, Heredia-Elvar JR, Fortes LS,
Silva-Grigoletto ME. Reproducibility of inhibitory control, working memory, and cognitive
flexibility measures in older women. Rev Bras
Fisiol Exerc 2023;22:e225470. doi: 10.33233/rbfex.v22i1.5470
Abstract
Introduction: Executive Function is expressed in day-to-day
activities through inhibitory control, working memory, and cognitive
flexibility. Despite the importance of evaluating these measures, there are
disagreements about the reproducibility of the tests. Objective: To test
the reproducibility of the Stroop Color-Word Test, Corsi
Block-Tapping Test, and Trail Making Test in older women. Methods:
Thirty-five older women performed the Stroop Color-Word Test (Inhibitory
Control), Corsi Block-Tapping Test (Working Memory),
and Trail Making Test (Cognitive Flexibility) within one week between the test
and retest. The reproducibility of the tests was determined by the intraclass
correlation coefficient, coefficient of variation, standard error of
measurement, and visual inspection of the Bland-Altman graphs. Results:
The Stroop Color-Word Test showed satisfactory reproducibility values only for
congruent and incongruent measures, with excellent intraclass correlation
coefficient values. Corsi Block-Tapping Test showed
reproducible values with a moderate and good intraclass correlation coefficient
for the sequence and composite score, respectively. The Trail Making Test
showed reproducible values for parts A, B, and the ratio (B/A), with intraclass
correlation coefficients between moderate and good. Visual inspection of the
Bland-Altman plots showed low bias in all variables. Conclusion: The
results of the Stroop Color-Word Test, for congruent and incongruent trials,
the sequence and the composite score of the Corsi
Block-Tapping Test, as well as the part A, B, and the ratio (B/A) of the Trail
Making Test, are reproducible measurements for older women.
Keywords:
test-retest reliability; executive function; old people; neuropsychological
tests.
Resumo
Introdução: A
Função Executiva é expressa nas atividades do dia a dia por meio do controle
inibitório, memória de trabalho e da flexibilidade cognitiva. Apesar da
importância de avaliar essas medidas, existem divergências sobre a
reprodutibilidade dos testes. Objetivo: Testar a reprodutibilidade do Stroop Color-Word Test, Teste dos Cubos de Corsi e Teste de
Trilhas em mulheres idosas. Métodos: Trinta e cinco mulheres idosas
realizaram o Stroop Color-Word Test (Controle
Inibitório), Teste dos Cubos de Corsi (Memória de Trabalho) e Teste de Trilhas
(Flexibilidade Cognitiva) com uma semana entre o teste e reteste. A reprodutibilidade
dos testes foi determinada pelo coeficiente de correlação intraclasse,
coeficiente de variação, erro padrão da medida e inspeção visual dos gráficos
de Bland-Altman. Resultados: O Stroop Color-Word Test apresentou valores satisfatórios quanto
à reprodutibilidade apenas para as medidas congruentes e incongruentes, com
valores excelentes de coeficiente de correlação intraclasse. O Teste dos Cubos
de Corsi apresentou valores reprodutíveis com coeficiente de correlação
intraclasse moderado e bom para a sequência e escore composto, respectivamente.
O Teste de Trilhas apresentou valores reprodutíveis para as partes A, B e a
razão (B/A), com coeficientes de correlação intraclasse entre moderado e bom. A
inspeção visual nos gráficos de Bland-Altman
demonstrou baixo viés em todas as variáveis. Conclusão: Os resultados do
Stroop Color-Word Test, para ensaios congruentes e
incongruentes, a sequência e o escore composto do Teste dos Cubos de Corsi,
assim como a parte A, B e a razão (B/A) do Teste de Trilha são medidas
reprodutíveis para mulheres idosas.
Palavras-chave:
confiabilidade do teste-reteste; função executiva; pessoas idosas; testes
neuropsicológicos.
Introduction
Executive Function (EF) is about
higher mental processes that ensure a person engages in day-to-day behaviors
[1]. EF includes necessary skills when attentional resources are required
throughout a task, in addition to being used for automatic and intuitive
cognitive processes [1]. It allows the individual to reflect before acting,
work on different ideas, solve unexpected challenges, think from different
perspectives, reconsider divergent opinions, and avoid distractions [2]. The
proper functioning of the EF is essential for maintaining the quality of life
[3,4]. Among the EF domains, the most studied are inhibitory control, working
memory, and cognitive flexibility.
Inhibitory control is responsible
for inhibiting mental and behavioral processes to the detriment of an
objective, such as adapting actions to external objections; for example, in a
conversation, we do not say everything we think and feel. It is necessary to
choose what to say according to the social context [5]. Working memory, in
turn, is seen as the manipulation of memory according to the required demand;
for example, when cooking according to a recipe, it is necessary to follow
steps properly to achieve the desired result [6]. Finally, cognitive
flexibility is the mental process related to adapting to challenges or events,
being used to make adjustments to previously planned actions or to create
something in a context; for example, when we have several options and need to
choose only a few of them to achieve a result [1].
The literature presents several
tasks to assess inhibitory control. The most popular ones are the Go/No-Go
paradigms [7], the Flanker task [8], and the Stroop Color-Word Test (SCWT) [9].
The Go/No-Go is a task with different stimuli, some that must be answered and
some that must not. For example, the subject must react when viewing an arrow
to the right, while he must not react to seeing an arrow to the left [10]. The
Flanker task, in turn, is based on the use of sets of arrows or symbols that
can be congruent (e.g., all arrows in the same direction
“<<<<<”), incongruent (e.g., different directions “>>
<>>”), or neutral (e.g., including arrows and other symbols
“---<--”) [8]. Finally, the most common is the SCWT, which is based on names
of colors that are filled in by the same color as the word indicates (congruent)
or a different color (incongruent), and the subject must indicate the filling
color, not inhibiting the reading of the which is written [9]. The SCWT has a
vast literature, but there are divergences regarding the scoring and
reproducibility of this test [11,12,13,14]. In this sense, it is necessary to
evaluate the reproducibility of the SCWT in a computerized way in elderly
individuals, standardizing its form of execution and scoring.
Working memory, in turn, can be
assessed through verbal or non-verbal tasks. The N-back test explores verbal
and non-verbal tasks, while the Corsi Block-Tapping
Test (CBTT) is non-verbal [15,16,17]. In the N-back test, the individual must
remember previous numbers or images, which can be called 1-back (remembering
the displayed number before the current number), 2-back (remembering the
displayed number before the last two numbers presented), and so on, making it
possible to assess both response time and accuracy [15]. The CBTT assesses
visuospatial working memory, asking the participant to select squares in the
same order in which they were presented (direct order) or in reverse order,
starting from the last square presented to the first. In the CBTT, it is
possible to evaluate the composite score (sequence x number of correct answers)
or only the sequence of correct answers. However, the literature still differs
on the best score to be adopted, besides not presenting good reproducibility
values even when performing six tests with one-week intervals, mainly with
older people [18,19,20].
Cognitive flexibility is understood
as a result of inhibitory control and working memory since it is necessary to
inhibit a premeditated action (inhibitory control) and check alternatives to
act differently compared to previous experiences (working memory) [1]. The Trail
Making Test (TMT) and the Wisconsin Card Sorting Task are two approaches to
assessing cognitive flexibility [1,21,22]. In the Wisconsin card sorting task,
the participant must match cards from a deck totaling 128 with four target
cards dealt on the table. Cards can be combined based on their colors “red,
blue, yellow or green” or geometric shapes “crosses, circles, triangles or
stars”. The test combines ten cards based on colors or geometric shapes [23].
The TMT, in turn, consists of a task divided into two parts, A and B. The TMT-A
assesses the processing speed by considering the time the participant uses to
connect 25 dots in ascending numerical order. The TMT-B represents the visual
search and the cognitive flexibility when evaluating the connection of numbers,
and letters in ascending and intercalated order (e.g., a number and a letter)
arranged randomly. Thus, the TMT-B includes inhibitory control when verifying
the non-linking of a letter with a letter or number with a number and working
memory when needing to remember the increasing numerical and alphabetic
sequence after each connection. Among the ways of analyzing the TMT score is
the difference (B-A) and the ratio (B/A) in the execution time [19,24,25]. In
this sense, the study by Wang et al. [25] showed moderate
reproducibility for TMT-A and excellent reproducibility for TMT-B in elderly
individuals. However, they do not address other measures such as the difference
(B-A) and the ratio (B/A), in addition to the fact that the literature does not
present a consensus on its use for the public of older women and the interval
between test and retest applications.
In this sense, it is necessary to
analyze what is more relevant considering the evaluation of EF: evaluating only
one domain in isolation or applying different tests to different domains.
Consequently, the application of various EF tests in sequence, as well as the
reapplication interval and target audience, may affect the reproducibility of
EF tests. Therefore, we aimed to test the reproducibility of SCWT, CBTT, and
TMT in older women sequentially using a seven-day interval between
measurements. We believe that, when considering the sample involved in the
study, seven days is the most appropriate to minimize the learning effect and
ensure better reproducibility in the tests. Additionally, we believe that even
when applied sequentially, the tests will present good reproducibility compared
to the values shown in the literature, allowing a consistent evaluation of the
main EF domains.
Methods
Participants
A total of 70 women were recruited
through leafleting around the Prof. José Aloísio de
Campos campus from the Federal University of Sergipe in São Cristovão.
Inclusion criteria were: having at least 12 Montreal Cognitive Assessment
(MoCA) points; being physically independent; being aged between 60 and 79
years; being literate. In turn, the exclusion criteria were: having color
blindness; neurological and/or psychiatric disorders (e.g., Parkinson's
disease); hearing or visual impairment incompatible with the neuropsychology of
the tests; and not having a fine motor impairment that could interfere with the
performance of cognitive and motor tasks.
After
the screening, 40
participants met the inclusion criteria, and 35 participants performed
the
three tests proposed in the study sequentially and with an interval of
seven
days between the test and the retest (Figure 1). Before data
collection, the
participants signed the informed consent form (TCLE) after explaining
all the
procedures. The research was submitted to the institution’s
ethics committee, approved under opinion 3.225.938, and followed the
Declaration of Helsinki for research with human beings.
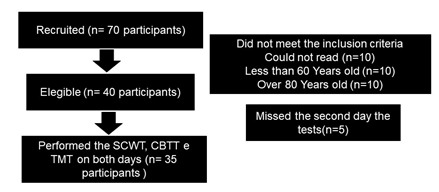
Figure
1 - Participants' flowchart
Executive Function Protocol
Initially, body mass and height
measurements were obtained to calculate the body mass index (BMI). The MoCA
questionnaire was applied, which involves EF, visuospatial
working memory, episodic memory, and attention to assess the global cognition
of older people [26,27].
Each participant visited the
laboratory in three different sessions: the first for sample characterization
and two with an interval of seven days between them to perform the tests in the
morning. Each session lasted 30 minutes. Aiming to keep the participants, a
reminder was given three days before the sessions to confirm participation.
Before the measurements, the participants were familiarized with the devices
used to carry out the tests.
On the day before the tests, the
participants were instructed through a call and message to abstain from alcohol
and vigorous physical activity for 24 hours, in addition to not smoking or
ingesting caffeine within two hours before the experiment. The tests were
conducted between March and November 2022 and were always applied by the same
evaluator.
The SCWT and CBTT tests were
performed on computers with a 15-inch screen. The PsychoPy®
program version 2022 1.3 (https://www.psychopy.org/) was used to build the
stimuli and set up the experiment, and it was made available online through the
Pavlovia platform (https://pavlovia.org/). The
participants used keyboards with yellow, blue, green, and red stickers on the A,
D, J, and L keys to perform commands during the tests.
The participant rested for five
minutes before the tests, and then the tests started. For this, the participant
remained seated, facing a monitor at a distance of 50 cm. Then, the tests were applied
in the following order: SCWT, CBTT, and TMT. Instructions for each task were
provided verbally and in writing on the computer screen.
Stroop Color-Word Test (SCWT)
SCWT assesses inhibitory control
[11]. The test has congruent (word meaning equal to its font color) and
incongruent (word meaning and font color divergent) responses. First, the
participant performed 10% of the trials for familiarization with the
experiment, resulting in 12 trials out of 120. Then, the participants completed
120 trials, 60 congruent and 60 incongruent. During the test, participants were
asked to respond as quickly as possible. The response time (RT) for congruent
stimuli and the RT for incongruent stimuli that expresses inhibitory control
were analyzed. Furthermore, we analyzed the mean difference in performance
between congruent and incongruent trials, commonly called the Stroop effect,
which is yet another measure of inhibitory control [14]. The test was
considered valid when the participant obtained an accuracy of at least 80%.
Corsi Block-Tapping Test (CBTT)
This test evaluates visuospatial
working memory [19]. At the beginning of the test, there were four
familiarization trials with only two squares, in which they got hit-or-miss
feedback. Our test consisted of nine squares (2 cm x 2 cm) in blue, and every
500 ms, a square changed color, turned yellow, and
then returned to blue at random. Then, the participant was asked to indicate
which changed color in the same order in which the changes occurred (direct
order). The participants received no feedback regarding the successes and
errors in the test. If the participant got the sequence right, the test
progressed by increasing the number of squares. On the other hand, if the
participant made a mistake twice in a row, the test was terminated. In this
test, the applicator helped the participants by using the mouse to select the
sequence they indicated since they were unfamiliar with the mouse. The values
referring to the sequence the participant reached in a given trial and the
composite score calculated by multiplying the number of correct answers
obtained in all trials by the sequence score were used for analysis.
Trail Making Test (TMT)
This test assesses cognitive
flexibility [22]. The TMT consisted of two parts: in part A, participants were
asked to continuously call, using a ballpoint pen, numbers from 1 to 25
randomly arranged on a sheet of paper. In part B, participants were asked to
continuously connect numbers and letters alternately (e.g., 1-A, 2-B, etc.).
The score on both parts is defined by the time to run the test correctly. Then,
the difference (B-A) is taken as an index of cognitive flexibility, and the
higher the score, the lower the participant's cognitive flexibility [28]. In
addition, the ratio (B/A) was calculated, which is also an estimate of
cognitive flexibility. In the test application, we followed Reitan's
recommendation [22], in which errors were not accounted for. In case of error,
the evaluator indicated that the participant returned to the last number or
letter and continued the test [28].
Statistical analysis
The sample size was calculated
using the G*Power 3.1.9.7 software based on an unpublished pilot study,
considering an alpha error of 0.05, power of 0.95, and the ratio between the
alternative and null hypothesis equivalent to 0,35 resulting in a minimum
sample of 27 participants [29,30]. This sample calculation method was
previously used by Fontes et al. [31]. All data were analyzed using the
JAMOVI software, version 2.3.16. Data normality was tested using the
Shapiro-Wilk test. The reproducibility of SCWT, CBTT, and TMT was determined by
the two-way intraclass correlation coefficient (ICC). The ICC was interpreted
according to the Koo et al. [32] classification system for
reproducibility: < 0.50 = poor; 0.50-0.75 = moderate; 0.75-0.90 = good; and
> 0.90 = excellent. In addition, the coefficient of variation (CV) and
standard error of measurement (SEM) were calculated. The level of agreement between
sessions was analyzed using the Bland-Altman plot, considering the systematic
bias and its limits of agreement of 95% (LoA = Bias) [33]. Additionally, data on the sum of the differences between the
means on the two evaluation days were analyzed to visualize the agreement
between the measurements better. Graphs were constructed using GraphPad Prism
software version 8.
Results
Namely, the sample analyzed had an
average age of 66.4 ± 5.4 years, a body mass of 67.1 ± 11.5 kg, a height of 1.55
± 0.05 m, and a BMI of 28.0 ± 4.2 kg/m2. In addition, the
participants had an average score of 21.9 ± 3.83 points on the MoCA.
Regarding the Congruent and
Incongruent RT of the SCWT, an excellent ICC, low CV, and SEM within the
expected range were observed (Table I). We detected low bias for the two
measures based on the agreement analysis with only two individuals beyond the
agreement interval (Figure 2). Regarding SE, we observed a moderate ICC and SEM
within the expected range but a high CV (Table I). In addition, the agreement
between measurements showed a bias close to zero, and only three individuals
were outside the limits of agreement (Figure 2).
Regarding the CBTT, the sequence
analysis results showed moderate ICC, low CV, and SEM within the expected range
(Table I). There was a bias close to zero in the agreement between
measurements, and only one individual exceeded the limits of agreement (Figure
3). The composite score demonstrated a good ICC, low CV, and within the
expected SEM (Table I). Finally, the agreement between the measures had a bias
close to zero, and only one individual was outside the limits of agreement
(Figure 3).
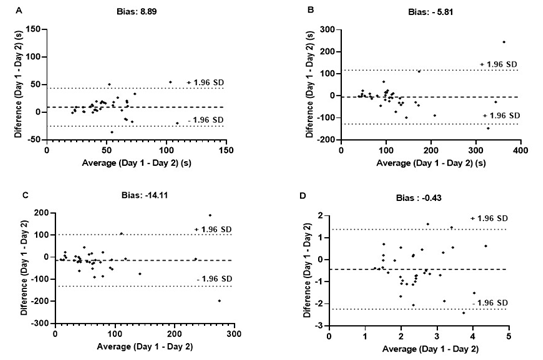
Regarding TMT-A and TMT-B, a good
ICC, low CV, and SEM within the expected range were verified (Table I).
Regarding the agreement between measurements, we found a bias close to zero in
both variables, with two individuals exceeding the limit of agreement in the
TMT-B (Figure 4). Using other measures of cognitive flexibility, specifically,
the difference (B-A), good ICC, high CV, and within expected SEM were observed
(Table I). The agreement between measurements showed a bias close to zero with
two individuals outside the agreement limit. In the ratio (B/A), a moderate
ICC, low CV, and SEM within the expected range were detected (Table I). The
agreement between measurements showed a bias close to zero, and only one
individual was outside the limits of agreement (Figure 4).
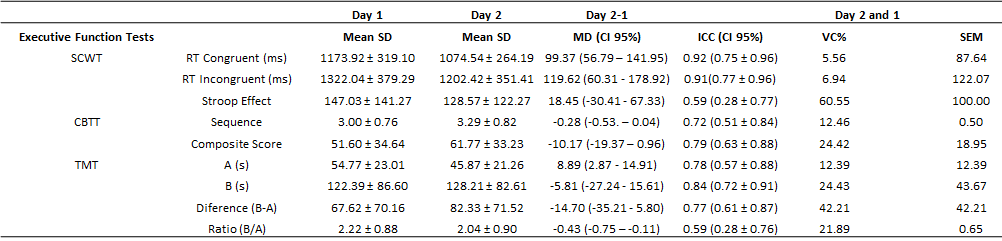
SD
= Standard Deviation; MD = Mean Difference; CI = Confidence Interval; ICC =
Intraclass Correlation Coefficient; CV = Coefficient of Variation; SEM =
Standard Error of Measurement; RT = Response Time; SCWT = Stroop Color-Word
Test; TMT = Trail Making Test; CBTT = Corsi
Block-Tapping Test
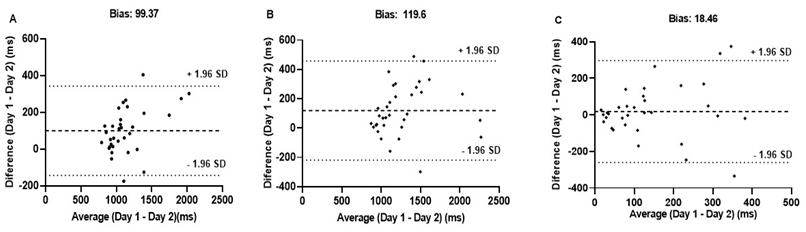
Figure
2 - Bland-Altman plots of differences between Day 1
and Day 2 as a function of the mean of paired measurements for RT Congruent (A)
and RT Incongruent (B) and the Stroop effect (C). The dotted line represents
the systemic bias, and the dashed lines represent the upper and lower limits of
agreement
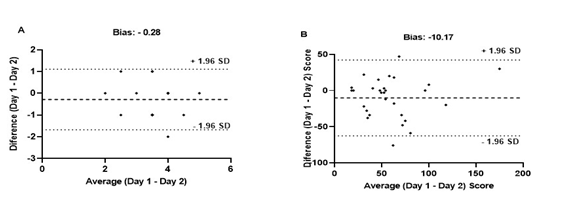
Figure
3 - Bland-Altman plots of differences between Day 1
and Day 2 as a function of the mean of paired measures for the CBTT sequence
(A) and the CBTT composite score (B). The dotted line represents the systemic
bias and the dashed lines represent the upper and lower limits of agreement
Figure
4 - Bland-Altman plots of differences between Day 1
and Day 2 as a function of the mean of paired measurements for TMT-A (A), TMT-
B (B), difference (B-A) (C), and ratio (B/A) (D). The dotted line represents
the systemic bias and the dashed lines represent the upper and lower limits of
agreement
Discussion
The present study’s findings
partially corroborate our hypothesis since some of the results obtained in each
test were reproducible in older adult women. RT congruent and incongruent
results for the SCWT, composite score values for the CBTT, and the TMT-A,
TMT-B, and ratio (B/A) measures. Furthermore, the time interval used and the
application of the tests in sequence do not affect the reproducibility of the
measurements. Thus, our findings help outline research investigating the EF of
older women [34].
In the concordance analyses, we
found excellent reproducibility in the SCWT congruent and incongruent RT, low
CV, and low bias. However, the Stroop effect showed moderate reproducibility
and high CV. These findings corroborate those presented by Wang et al.
[24], who evaluated the reproducibility in older people in the congruent and
incongruent RT and demonstrated a value classified as excellent (ICC = 0.91)
with a period between the test and retest of three to seven days.
Interestingly, Wang et al. [24] applied the SCWT using pencil and paper
while we performed it using computers. Thus, there may be no significant impact
on the measurement of inhibitory control with different application forms.
However, the application through computers makes it easier from the application
to the evaluation and number of tests applied [20,35]. These findings apply to
older women since other studies with young adults found values below those
presented in the present study [12].
Regarding the CBTT values, the
sequence and the composite score were analyzed, demonstrating that both
variables have good reproducibility. These values differ from the study by
White et al. [20] in which direct order CBTT was applied to 30 healthy
older men, showing poor reproducibility in sequence and composite score
measures [20]. A possible explanation may be given by the help of the
applicator in handling the mouse, which is an important aspect when considering
the application of this test in a computerized way to guarantee the quality of
the measurement since the older adult population tends to present deficits in
fine motor control and low familiarization with the use of the mouse [36].
Regarding cognitive flexibility,
the values referring to TMT-A, B, and difference (B-A) presented a good classification
in the ICC. In contrast, the ratio (B/A) showed a moderate ICC. It is also
important to note that the CV for TMT-A, TMT-B, and the ratio (B/A) were
classified as low. These findings partially corroborate with other studies that
analyzed the same population, such as the findings of Park and Shott [37], who
evaluated TMT-A and TMT-B measurements in older people, finding an excellent
ICC. However, in these studies, the authors considered individuals 50 years old
as older people. Another study applying the Chinese version of the TMT
addressed test reproducibility in older people and demonstrated a good ICC in
TMT-A and excellent in TMT-B using an evaluation interval similar to that of
the present study, from three to seven days [25]. A possible reason for the
differences is the diversity of education in the sample between the studies
since we do not require a minimum education level. Another important point of
our study is the standardization of the interval between applications. It is
also worth mentioning that we maintained the performance of this test with pen
and paper since the literature recommends the application in this way [38,39].
Although the tests used alone are
reported in the literature as general indicators of EF, each assesses a domain
in isolation. A strength of our study was an integrated approach, using SCWT to
assess inhibitory control, CBTT for working memory, and TMT to assess cognitive
flexibility, thus favoring the interpretation of the global state of EF [11].
In turn, we adopted the application of SCWT and CBTT in a computerized way
based on free access protocols and software, which facilitates the method of
reproduction used in clinical practice and scientific research. In addition to
innovating by bringing the reproducibility of neurocognitive tests in a
computerized format in older people, this is relatively scarce in the
literature [20]. Thus, our findings provide important insights for a
comprehensive assessment and follow-up of EF in older women.
Among the limitations of the
present study, we can point out the possibility of the learning effect since
only two measurements were performed for the test and retest. However, we
believe that the seven-day interval between measurements minimizes this effect.
Furthermore, to reduce the learning effect, the SCWT and CBTT tests were
planned with the sequences of words and blocks randomized between the test and
retest days.
Another limitation is the small
sample size, which may increase the chance of type I or II error, although we
met our sample calculation. In this sense, the literature has no consensus
about the best way to calculate sample size for reproducibility studies.
Furthermore, most studies used two groups, and we used only one. Thus, there
may be differences compared to other groups. Anyway, considering the normality
of the data, we believe that the results observed in the present study
contribute to the literature regarding tests for EF in older
women since we provide detailed information on the characteristics of the
tasks, instructions, stimuli, and scoring methods, presenting itself as an
important differential for other studies in the area [40]. In addition, we
provide score values that can be considered in other scientific studies and clinical
practice.
Conclusion
The evaluation of the congruent and
incongruent RT in the SCWT for inhibitory control, the sequence and a composite
score of the CBTT for visuospatial working memory, and the TMT-A, TMT-B and the
ratio (B/A) in the TMT for cognitive flexibility are reproducible methods for
assessing EF in older women. In addition, carrying out the tests sequentially
and with an interval of one week is an effective approach to guarantee the
reproducibility of these evaluations.
Academic
affiliation
This
article represents part of Alan Pantoja Cardoso's Master's thesis, supervised
by Professor Dr. Marzo Edir
Da Silva-Grigoletto, from
the Federal University of Sergipe, Brazil.
Conflict
of interest
There
is no conflict of interest
Funding
source
Part
of this study is funded by the Coordination for the Improvement of Higher
Education Personnel - Brazil (CAPES)
Authors’ contributions
Research conception
and design: Pantoja-Cardoso A, Faro HKC; Data collection: Pantoja-Cardoso A, Dos-Santos AC, Santos
PJ; Data analysis and interpretation: Pantoja-Cardoso A, Aragão-Santos JS; Manuscript writing:
Pantoja-Cardoso A, Aragão-Santos JS, Monteiro MCP, Santos PJ, Heredia-Elvar JR, Dos-Santos AC; Critical
review of the manuscript for important intellectual content: Fortes
LS, Da Silva-Grigoletto ME
- Diamond A. Executive Functions. Annu Rev Psychol. 2013;64:135-68. doi: 10.1146/annurev-psych-113011-143750 [Crossref]
- Diamond A. Why improving and assessing EF early in life is critical. In: Griffin JA, McCardle P, Freund LS, eds. Executive function in preschool-age children: Integrating measurement, neurodevelopment, and translational research. American Psychological Association 2016;11-43. doi: 10.1037/14797-002 [Crossref]
- Harada CN, Love MCN, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737-52. doi: 10.1016/j.cger.2013.07.002 [Crossref]
- Ho H-T, Lin S-I, Guo N-W, Yang YC, Lin MH, Wang CS. Executive function predict the quality of life and negative emotion in older adults with diabetes: A longitudinal study. Prim Care Diabetes. 2022;16:537-42. doi: 10.1016/j.pcd.2022.05.002 [Crossref]
- Aron AR, Robbins TW, Poldrack
RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18(4):177-85. doi: 10.1016/j.tics.2013.12.003 [Crossref]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193-224. doi: 10.1146/annurev.psych.59.103006.093615 [Crossref]
- Donders FC. On the speed of mental processes. Acta Psychol (Amst). 1969;30:412-31. doi: 10.1016/0001-6918(69)90065-1 [Crossref]
- Servant M, Logan GD. Dynamics of attentional focusing in the Eriksen flanker task. Atten Percept Psychophys. 2019;81(8):2710-2721. doi: 10.3758/s13414-019-01796-3 [Crossref]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology 1935;18:643-62. doi: 10.1037/h0054651 [Crossref]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal
paradigm. Trends Cogn Sci 2008;12:418-24. doi: 10.1016/j.tics.2008.07.005 [Crossref]
- Scarpina F, Tagini S. The stroop color and word test. Front Psychol 2017;8:1-8. doi: 10.3389/fpsyg.2017.00557 [Crossref]
- Strauss GP, Allen DN, Jorgensen ML, Cramer SL. Test-retest reliability of standard and emotional stroop tasks: an investigation of color-word and picture-word versions. Assessment. 2005;12(3):330-7. doi: 10.1177/1073191105276375 [Crossref]
- Periáñez JA, Lubrini G,
García-Gutiérrez A, Ríos- Lago M. Construct validity of the stroop
color-word test: influence of speed of visual search, verbal fluency, working
memory, cognitive flexibility, and conflict monitoring. Arch Clin Neuropsychol. 2021;36(1):99-111. doi: 10.1093/arclin/acaa034 [Crossref]
- Ward N, Hussey E, Alzahabi
R, Gaspar JG, Kramer AF. Age-related effects on a novel dual-task Stroop
paradigm. PLoS ONE 2021;16:e0247923. doi: 10.1371/journal.pone.0247923 [Crossref]
- De Nardi T, Sanvicente-Vieira B,
Prando M, Stein LM, Fonseca RP, Grassi-Oliveira R. Tarefa N-back
Auditiva: Desempenho entre diferentes grupos etários. Psicol Reflex Crit. 2013;26(1). doi: 10.1590/S0102-79722013000100016 [Crossref]
- Gonçalves VT, Mansur LL. N-Back auditory test performance in normal individuals. Dement Neuropsychol. 2009;3(2). doi: 10.1590/S1980-57642009DN30200008 [Crossref]
- Nyberg L, Dahlin E, Stigsdotter Neely A, et al. Neural correlates of variable working memory load across adult age and skill: Dissociative patterns within the fronto-parietal network. Scand J Psychol. 2009;50(1):41-6. doi: 10.1111/j.1467-9450.2008.00678.x [Crossref]
- Arce T, McMullen K. The Corsi
Block-Tapping Test: Evaluating methodological practices with an eye towards
modern digital frameworks. Computers in Human Behavior Reports [Internet]. 2021
[cited 2022 dez 12];4:100099.
Available from: https://par.nsf.gov/servlets/purl/10292489
- Corsi PM. Human memory and the medial temporal region of
the brain [Internet]. Dissertation Abstracts International 1973;34(2-B): 891.
Available from: https://psycnet.apa.org/record/1976-04900-001
- White N, Flannery L, McClintock A, Machado L. Repeated computerized cognitive testing: Performance shifts and test–retest reliability in healthy older adults. J Clin Exp Neuropsychol 2019;41:179-91. doi: 10.1080/13803395.2018.1526888 [Crossref]
- Milner B. Some cognitive effects of frontal-lobe
lesions in man. Philos Trans R Soc Lond B Biol Sci. 1982 Jun 25;298(1089):211-26. doi: 10.1098/rstb.1982.0083 [Crossref]
- Reitan RM. The relation of the Trail Making Test to organic
brain damage. J Consult Psychol.
1955;19(5):393-4. doi: 10.1037/ [Crossref]
- Miguel
FK. Teste Wisconsin de Classificação de Cartas. Avaliação Psicológica 2005;4:203-4.
- Suzuki H, Sakuma N, Kobayashi M, Ogawa S, Inagaki H, Edahiro A, e t al. Normative data of the Trail Making Test among urban community-dwelling older adults in Japan. Front Aging Neurosci. 2022;14:832158. doi: 10.3389/fnagi.2022.832158 [Crossref]
- Wang R-Y, Zhou J-H, Huang Y-C, Yang Y-R. Reliability of the Chinese version of the Trail Making Test and Stroop Color and Word Test among older adults. Int J Gerontol. 2018;12:336-9. doi: 10.1016/j.ijge.2018.06.003 [Crossref]
- Cesar KG, Yassuda MS, Porto FHG, Brucki SMD, Nitrini R. MoCA Test: normative and diagnostic accuracy data for seniors with heterogeneous educational levels in Brazil. Arq Neuro-Psiquiatr. 2019;77(11). doi: 10.1590/0004-282X20190130 [Crossref]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-9. doi: 10.1111/j.1532-5415.2005.53221.x [Crossref]
- Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc 2006;1:2277-81. doi: 10.1038/nprot.2006.390 [Crossref]
- Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149-60. doi: 10.3758/BRM.41.4.1149 [Crossref]
- Shieh G. Sample size requirements for the design of reliability studies: precision consideration. Behav Res Methods. 2014;46(3):808-22. doi: 10.3758/s13428-013-0415-1 [Crossref]
- Fontes AS, Santos MS, Almeida MB, Marín PJ, Silva DRP, Silva-Grigoletto MES. Inter-day reliability of the Upper Body Test for shoulder and pelvic girdle stability in adults. Brazilian Journal of Physical Therapy 2020;24:161-6. doi: 10.1016/j.bjpt.2019.02.009 [Crossref]
- Koo TK, Li MY. A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine 2016;15:155-63. doi: 10.1016/j.jcm.2016.02.012 [Crossref]
- Martin Bland J, Altman Douglas G. Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet 1986;327:307-10. doi: 10.1016/S0140-6736(86)90837-8 [Crossref]
- Harvey PD. Domains of cognition and their assessment. Dialogues in Clinical Neurosci. 2019;21:227-37. doi: 10.31887/DCNS.2019.21.3/pharvey [Crossref]
- Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test–retest intervals. J Int Neuropsychol Soc. 2003;9(3):419-28. doi: 10.1017/S1355617703930074 [Crossref]
- Hoogendam YY, van der Lijn F, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, et al. Older age relates to worsening of fine motor skills: a population-based study of middle-aged and elderly persons. Front Aging Neurosci. 2014;6. doi: 10.3389/fnagi.2014.00259 [Crossref]
- Park S-Y, Schott N. The trail-making-test: Comparison between paper-and-pencil and computerized versions in young and healthy older adults. Appl Neuropsychol Adult. 2022;29(5):1208-20. doi: 10.1080/23279095.2020.1864374 [Crossref]
- Miller JB, Barr WB. The technology crisis in neuropsychology. Arch Clin Neuropsychol. 2017;32:541-54. doi: 10.1093/arclin/acx050 [Crossref]
- Rumpf U, Menze I, Müller NG,
Schmicker M. Investigating the potential role of ecological validity on
change-detection memory tasks and distractor processing in younger and older
adults. Front Psychol. 2019;10:1046. doi: 10.3389/fpsyg.2019.01046 [Crossref]
- Zanini GAV, Miranda MC, Cogo-Moreira H, et al. An Adaptable, open-access test battery to study the fractionation of executive-functions in diverse populations. Front Psychol. 2021;12:627219. doi: 10.3389/fpsyg.2021.627219 [Crossref]
