Rev Bras Fisiol Exerc. 2024;23:e235475
doi: 10.33233/rbfex.v23i1.5475
ORIGINAL ARTICLE
Influence of dynapenic obesity on inflammation and muscle quality
in oldest-old patients
Influência da obesidade dinapênica sobre a inflamação e qualidade muscular em
pessoas idosas longevas
Bruno Viana Rosa1,
Danielle Garcia1, Ivo Vieira de Sousa Neto2, Vicente
Paulo Alves1, Lidiane Renata Ferreira de Oliveira de Paiva1,
Karla Helena Coelho Vilaça e Silva1, Flávia Maria Campos de Abreu1,
Dahan da Cunha Nascimento1
1Universidade Católica de Brasília (UCB),
Brasília, DF, Brasil
2Universidade de São Paulo (USP), São
Paulo, Brasil
Received; May 29, 2023; accepted
October 15, 2023
Correspondência: Dahan da Cunha Nascimento, dahanc@hotmail.com
Como citar
Rosa BV, Garcia D,
Sousa Neto IV, Alves VP, Paiva LRFO, Vilaça e Silva KHC, Abreu FMC, Nascimento
DC. Influence of dynapenic obesity on inflammation and muscle quality
in oldest-old patients. Rev Bras Fisiol
Exerc. 2023;1:e235475 doi:
10.33233/rbfex.v23i1.5475
Abstract
Introduction: Aging is associated with
a decrease in strength (dynapenia) and an increase in body fat. Both obesity and dynapenia negatively
influence health in the oldest old.
When these two variables coexist, called dynapenic obesity (DO), these further harms the
older adult's health. Objective: To verify the
influence of dynapenic obesity on inflammation, physical performance, and muscle quality in the oldest old
with and without DO. Methods: Forty-six oldest old participants were included in this study, and
sociodemographic, muscle strength, body composition, physical performance, and cytokine data were collected. The sample was divided into DO and control groups
to compare the variables. Results: The DO
group had higher levels of
inflammation, lower levels of absolute
handgrip strength, and field muscle
quality index than the control group,
but with no difference in physical
performance or laboratory muscle quality index. Conclusion: Therefore, this study points to dynapenic obesity
as an important variable that should
be evaluated and considered in the oldest old
to prevent possible adverse outcomes in this population.
Keywords: muscle strength; aging; obesity and inflammation.
Resumo
Introdução: O envelhecimento está associado à
diminuição da força (dinapenia) e ao aumento da
gordura corporal. Tanto a obesidade quanto a dinapenia
influenciam negativamente a saúde dos longevos. A coexistência dessas duas
variáveis, denominada obesidade dinapênica (OD),
prejudica ainda mais a saúde da pessoa idosa. Objetivo: Verificar a
influência da obesidade dinapênica na inflamação,
desempenho físico e qualidade muscular em pessoas idosas longevas com e sem OD.
Métodos: Foram incluídos 46 idosos longevos e coletados dados
sociodemográficos, de força muscular, composição corporal, desempenho físico e
citocinas. A amostra foi dividida em grupos OD e controle para comparar as
variáveis. Resultados: O grupo OD apresentou maiores níveis de
inflamação, níveis inferiores de força muscular absoluta de preensão manual e
índice de qualidade muscular de campo do que o grupo controle, mas sem
diferença no desempenho físico ou no índice de qualidade muscular laboratorial.
Conclusão: Portanto, este estudo aponta a obesidade dinapênica
como uma variável importante que deve ser avaliada e considerada em pessoas
idosas longevas para prevenir possíveis desfechos adversos nessa população.
Palavras-chave: força muscular; envelhecimento;
obesidade; inflamação.
Introduction
Most of the time, aging
is linked to unfavorable changes in body composition, with increased body fat up to
a certain age and a constant decline in lean muscle mass [1,2]. This accumulation of body fat can
lead to obesity, an inflammatory disease with an
increased risk for chronic diseases, including type 2 diabetes, hypertension, coronary heart disease, heart disease, and osteoarthritis [3]. Also, the decrease
in lean mass, which is associated
with several factors such as changes in the nervous system, the size and percentage
of type II fibers [4], and fat infiltration into the muscle
[5,6] contribute to lower muscle strength
(dynapenia) [7,8,9] and
sarcopenia [10]. Both obesity and
dynapenia negatively affect the health
and physical performance of older adults
[3], and the coexistence of these two factors
is called dynapenic obesity (OD) [11].
Another common characteristic
of aging is a dysregulation of the immune
system; this system typically
increases inflammation when needed and
decreases it when it is no longer. However,
when this inflammation is not removed and
remains in the long term, it can
result in pathologies
[12,13]. The breakdown of homeostasis of the immune system, which is usually
intrinsic to aging, causes chronic low-grade inflammation where levels of
pro-inflammatory cytokines,
such as tumor necrosis factor-α (TNF-α) and
IL6, are constantly increased
[14].
Both obesity and dynapenia
are also linked to chronic low-grade
inflammation [15,16], where
adipose tissue releases pro-inflammatory
cytokines. These cytokines cause several negative changes, such as increased expression of proteins that contribute to atrophy
of the skeletal
muscle [16,17] and decreased glucose uptake in this tissue favoring
the uptake of this substrate
in the adipose tissue, thus creating a vicious cycle of
muscle catabolism and increased body fat [15,18]. In addition, dynapenia and obesity
are factors associated with adverse health prognoses, such as frailty, all-cause mortality [19,20], inability to perform
activities of daily living [11], and lower physical performance
[21,22].
The presence of DO can be even
more harmful to the health of
older adults; this is confirmed
by studies that show that DO affects more than obesity or dynapenia
alone; several variables such as physical performance [8], increased
risk of falls
[23], mortality, hospitalization,
and disability [24]. In the oldest old
patients, there is difficulty in diagnosing DO because often, the term
DO is not known and little
explored [7]. Thus, patients with low
handgrip strength may be an
indication that should be considered
[7]. Moreover, deficits in the structure and
function of the intrinsic force-generating properties of skeletal muscle
are potential antecedents of dynapenia [7]. Thus, screening to clarify this
decline becomes an essential geriatric clinical parameter [8].
Furthermore, studies showed an association
between a pro-inflammatory condition and DO in the youngest-old and middle-old [25,26]. However, the literature
still lacks data on the oldest old
with and without DO and its relationship with inflammation and muscle quality. Therefore, this study aims to
verify the influence of DO on inflammation and muscle quality
in the oldest old participants.
Methods
Study design and participants
This observational,
descriptive, cross-sectional
investigation was part of a multicenter
study by the National Academic
Cooperation Program
(PROCAD). Only data referring to
the region of Distrito Federal were subject to statistical
tests. Data were considered if the
participant was considered an older
adult without hearing and/or
visual impairment who was able to
understand and respond to the
instruments applied. Those with orthostatic
intolerance or those with physical
disabilities that prevented independent walking were excluded.
After applying the inclusion and
exclusion criteria, 227 older adults were
eligible for evaluation. However, for cytokines analysis (the main
variables of this study), financial support was only
provided for 46 participants.
The variables analyzed in this study included
cytokines, gender, handgrip strength (HGS), physical performance, anthropometric
measurements (height and body weight), body composition, muscle quality index (MQI), dynapenia, number of medications,
diagnosis of systemic arterial hypertension, and diabetes mellitus (DM). This study was approved
by the local Institutional Research Ethics Committee (approval number:
50075215.2.0000.0029). The design and procedures were in accordance with ethical standards and the Declaration
of Helsinki. Each subject was fully
informed about the risks associated
with study participation and gave their written informed
consent.
Sociodemographic variables
For the adequate analysis
of the sociodemographic
variables, specific forms were used.
The older adult or his companion
filled out the identification form containing basic information such as full name, individual Taxpayer Registration Number, address, and telephone
contacts. The second part of the
form contained questions related to diagnosing pre-existing
diseases. The answers were filled out during the consultation
with the geriatrician and medical students, according to the information
provided on the referral sent
by a specialist doctor (e.g., cardiologist, pulmonologist, oncologist, endocrinologist, rheumatologist).
This information enabled the analysis
of the population's
age range and the identification of the most prevalent
diseases.
Handgrip strength measurement
Handgrip strength was recorded in kilograms/force (kg) using a duly calibrated hydraulic dynamometer (Lafayette Hydraulic Grip Dynamometer,
Lafayette Instruments Inc.) [27]. The participants were instructed to sit
in a chair with arms, keeping the
dominant arm at a 90° angle with the contralateral limb relaxed on
the thigh. During the measurements,
the evaluator provided verbal stimuli to encourage participants
to give their
best effort. Three consecutive measurements were performed with a one-minute rest interval interspersed. Also, the best
of the three
measurements was used in statistical analysis to encourage
the participants to get as high a score as possible [27].
Body composition
Body composition was analyzed using Dual Energy X-ray Absorptiometry (DXA) (Lunar,
model DPX-IQ, GE Lunar Corporation, pencil beam type, software version 4.7), which was properly calibrated
and operated by a trained professional. Participants were instructed to remove metal any accessories before lying in the supine position (feet together, arms slightly away from
the trunk and with the
wrists in a prone
position). The values for body composition
outcomes were determined from the ratio of
soft tissue attenuation of two X-ray
energy beams for each pixel containing a minimal amount of soft tissue but no significant bone [28]. Data collected from this assessment included information regarding total body fat and appendicular skeletal muscle mass (kg).
Appendicular skeletal muscle mass was
stratified into upper limb (UL-ASMM), lower limb (LL-ASMM), and total appendicular skeletal muscle mass (ASMM), which is defined by
the sum between upper and lower
limbs. Body fat is reported as
total body fat percentage
(%BF).
Muscle quality index
Laboratory MQI was determined by calculating
the ratio between HGS (kgf) and UL-ASMM
(kg) [29]. Field MQI was determined
by calculating the ratio between
HGS and BMI. The validity, reliability, and convenience of the MQI measures (field and laboratory)
have been previously reported [30,31,32].
Dynapenic obesity criteria
Prevalence of dynapenia was defined
by the handgrip
strength ≤ 27 kg, and
≤ 16 kg [33,34], for men and
women, respectively. Obesity was considered
a body fat percentage of ≥ 27% and ≥ 38%
[34], for men and women, respectively. DO was determined if participants
fulfilled the criteria for both dynapenia and obesity using these definitions.
The control group was considered participants who did not fulfill the criteria
cited above.
Physical performance test
To assess functional performance, the short
physical performance battery
(SPPB) was used. The battery is composed
of three tests: static balance, in three different standing positions, with increasing levels of difficulty;
walking speed, on a 3-meter course with the usual walking speed; and strength of
lower limbs, through the test
of sitting and getting up
from the chair five times, as quickly as possible. Each subtest is
scored on a scale from zero to four points, with twelve being the
total score [35].
Cytokines
Inflammatory profile was assessed using high-throughput flow cytometry (FACS Verse model; BD Biosciences,
San Jose, CA, USA) with the
serum previously collected and the
Human Th1/Th2 cytokine kit
as reagent (BD Biosciences)
to assess six mediators: IFNc, IL-2, IL-4, IL-6, IL-10, and
TNF-α. The reactions were
performed following the manufacturer’s protocol, producing a titration curve with standards provided by the
kit. All scores were estimated by interpolation
of the respective
curve. Whenever a given
sample yielded out of range
of outlying readings, the assay
was repeated with an original or diluted sample (as necessary) until a minimum of three
hundred events were acquired for each type of
cytokine bead used. All data were analyzed using
FCAP software, version 3.0 (BD Biosciences).
Statistical analysis
Data are mean ± standard deviation, unless otherwise stated. Normality was assessed by
Shapiro-Wilk’s test. However,
for cytokines variables,
non-normality was observed, and a logarithmic transformation was applied. An
independent-samples t-test was run to
determine if there were differences in DO and control group
in body composition, functional
performance, and muscle quality index. For cytokines a
Mann-Whitney U test was applied. A chi-square test (qui²)
was also performed to determine if an association
between groups, and diseases exists
and to analyze
the baseline characteristics
of the participants.
When expected cell frequencies were lower than five,
the Fisher’s Exact test was
used. For power analysis, considering a mean difference of 10 pg/ml between
groups for IL-6, and an effect size
of 0.63. A power of 0.50 was observed
for 44 participants (t-tests
– Means: difference between two independent
means). An alpha level of α ≤ 0.05 was considered significant. For data analysis,
SPSS (version 20.00) and
G*Power 3.1.6 [36] were used.
Results
Considering the financial
support for cytokine analysis, the final sample included 46 octogenarians.
Baseline characteristics of
the 46 participants are shown in Table I.
Table I shows that control group displayed
a lower body fat (p =
0.04), higher absolute hand-grip strength (p = 0.04), and a higher field
MQI (p = 0.007) compared to
the DO group. For other variables, no differences were observed (p > 0.05).
Table I - Participant’s characteristics with and without dynapenic
obesity
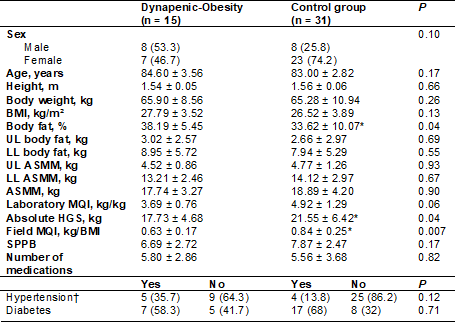
Result is
presented by mean and standard deviation; * significantly different between groups (p < 0.05); BMI = body mass
index; UL = upper limbs; LL
= lower limbs; ASMM = appendicular skeletal muscle mass; MQI = muscle quality index; HGS = handgrip strength; For chi-square test,
data is presented as frequency and percentage
values; †Fisher exact test
For cytokines, significant differences between groups were observed
for IL-6 levels (p = 0.005). The DO group displayed a higher IL-6 levels when compared to
control group. For other variables no differences were observed (p > 0.05). Figure 1.
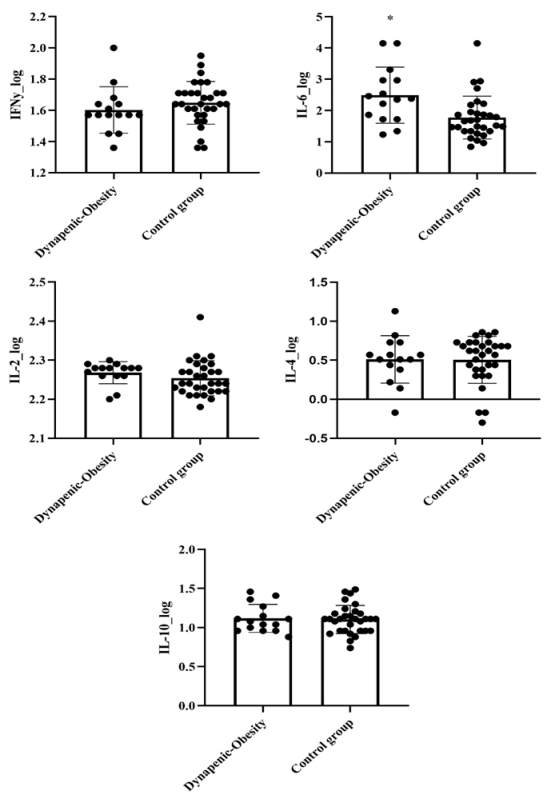
IL = interleukin, IFNy = Interferon gamma
Figure I - Data expressed
as mean and standard deviation (SD). *significant difference between groups (p < 0.05)
Discussion
This was the first research
to compare the oldest old participants
with and without dynapenic obesity. The result of this study
shows that the group oldest old
with DO had significantly higher levels of inflammation
(only for IL-6), lower levels of absolute
handgrip strength, and field MQI compared
to the control
group. However, no difference was found between groups
for physical performance measurement.
Also, a tendency for a significantly lower laboratory MQI for the DO group was verified.
However, no difference was found between
groups for physical
performance measurement (Figure 2).
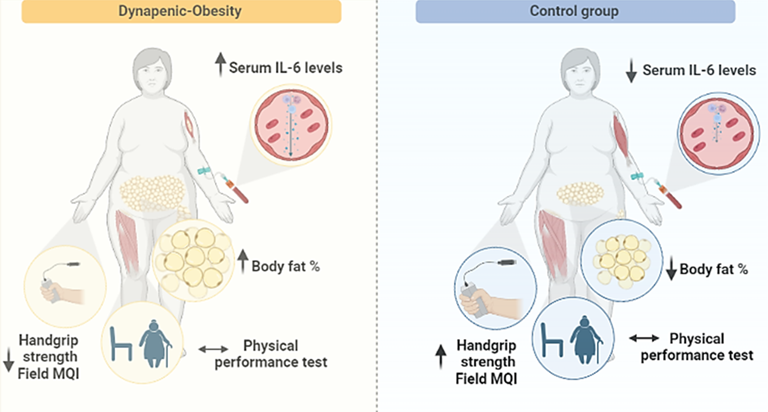
Figure 2 - Overview of
comparison between dynapenic-obesity and control group. increase (↑), decrease (↓) and no changes (↔)
The data on cytokines from
this research show that only IL-6 had higher levels
in the DO group, suggesting that this group might
display higher levels of inflammation compared to the
control group. In another study with
the oldest old, IL-6 was the
cytokine best associated, among several cytokines, with a higher metabolic
risk, low muscle strength, and gait speed
[37]. This may demonstrate the crucial role of IL-6 during aging. However, one study found
no difference in IL-6, IL-10, TNF-α, TNF-β,
and IL-1β between older adults with
DO or older adults with only
dynapenia [25] and another study showed
that between IL-6, IL-10, and TNF-α, only the last two
had lower and higher levels,
respectively, in the DO group compared to the obese,
non-obese, or low-strength group [26]. Similar previous research only examined the
role of the association of inflammatory markers in the youngest-old [25]. However, differences in results may be
related to differences in obesity assessment
methods, such as the use of BMI or differences in the research population
[25,26].
Another finding of this study
was a lower field MQI in the DO, but with no difference
between groups for the laboratory MQI, despite a tendency towards statistical significance. The literature
points out that obese people have a lower
MQI compared to non-obese people [38]. In this sense, there
are mechanisms that are linked to the
pathogenesis of DO, such as adipose tissue dysfunction (e.g., adipocyte hyperplasia and hypertrophy) [7]. In this condition, when fat is
accumulated in the form of intermuscular adipose tissue and intramyocellular
lipids, it can cause a lipotoxic effect, impairing the contractility
of muscle fibers and generating
lower strength and potency in the elderly [5,7] In addition, the infiltration
of adipocytes in muscle fibers implies
a lower neuromuscular activation
with low recruitment of motor units, reduction in the intrinsic contractile
capacity to generate force, and changes in actomyosin structure and function
[9]. But the data on the relationship
between MQI with DO is limited. However,
as the MQI is derived from a measure of strength
divided by a variable related to body mass [39], DO individuals are expected to present lower
values of muscle quality.
Another point is that, despite the
higher absolute value of the
SPPB test for the control group, we did not
find a significant difference between groups for performance in this research. These results do not corroborate with other studies that
showed lower performance on the SPBB test
in dynapenic abdominal obesity
older adults [8,40]. This difference between results may be due
to the reduced
sample in our study or to the
use of abdominal circumference
measurement in other studies [8,40]. This measurement of central obesity may better
reflect the distribution of fat in aging, as at this stage,
there is an accumulation of visceral fat with a drop in overall fat percentage, especially in the oldest old [2,12].
Finally, we address some limitations and future directions to improve the diagnosis of DO. The main limitation of this study
was the small
sample size due to financial restrictions for cytokine analysis in a more significant population. Furthermore, the cross-sectional design feature of
this study allows for only limited conclusions. Thus, more research is needed to
understand DO's inflammatory profile and
neuromuscular components.
Conclusion
In conclusion, dynapenic obesity oldest old displays a higher value of IL-6 and
lower values for Muscle Quality Index compared to oldest
old without dynapenic obesity.
Conflicts of interest
The authors declare that they have no conflict
of interest.
Financing source
The authors did not
receive any direct or indirect funding
for their research.
Authors' contribution
Research conception
and design: Nascimento DC, Abreu FMC, Neto IVS, Alves
VPA; Obtaining data: Rosa BV, Garcia D,
Paiva LRFO, Silva KHCV, Neto IVS; Data analysis and interpretation: Rosa BV,
Garcia D, Neto IVS; Statistical analysis: Nascimento DC; Writing
of the manuscript:
Rosa BV, Garcia D, Paiva LRFO, Silva KHCV; Critical
review of the manuscript for important intellectual content: Alves
VPA; Nascimento DC.
References
- Lee MM, Jebb
SA, Oke J, Piernas C. Reference values for skeletal muscle mass and fat
mass measured by bioelectrical impedance in 390 565 UK adults. J
Cachexia, Sarcopenia Muscle.
2020;11(2). doi: 10.1002/jcsm.12523 [Crossref]
- Strugnell C, Dunstan DW, Magliano DJ, Zimmet PZ, Shaw JE, Daly RM. Influence of age and gender on fat mass, fat-free mass and skeletal muscle mass among Australian adults: the Australian diabetes, obesity and lifestyle study (AusDiab). J Nutr Health Aging. 2014;18(5). doi: 10.1007/s12603-014-0464-x [Crossref]
- Stenholm S, Alley D, Bandinelli S, Griswold ME, Koskinen S, Rantanen T, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes (Lond). 2009;33(6). doi: 10.1038/ijo.2009.62 [Crossref]
- Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1). doi: 10.1002/jcsm.12238 [Crossref]
- Biltz NK, Collins KH, Shen KC, Schwartz K, Harris CA, Meyer GA. Infiltration of intramuscular adipose tissue impairs skeletal muscle contraction. J Physiol. 2020;598(13). doi: 10.1113/JP279595 [Crossref]
- Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports. 2011;21(6). doi: 10.1111/j.1600-0838.2011.01377.x [Crossref]
- Pérez-Campos Mayoral L, Matias-Cervantes CA, Pérez-Campos E, Romero Díaz C, Laguna Barrios LÁ, Pina Canseco MDS, et al. Associations of Dynapenic Obesity and Sarcopenic Obesity with the Risk of Complications in COVID-19. Int J Mol Sci. 2022;23(15). doi: 10.3390/ijms23158277 [Crossref]
- Máximo RO, Oliveira DC, Ramirez PC, Luiz MM, Souza AF, Delinocente MLB, et al. Combination of dynapenia and abdominal obesity affects long-term physical performance trajectories in older adults: sex differences. Am J Clin Nutr. 2022;115(5). doi: 10.1093/ajcn/nqac023 [Crossref]
- Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28(5). doi: 10.1016/j.nut.2011.12.002 [Crossref]
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3. doi: 10.3389/fphys.2012.00260 [Crossref]
- Yang M, Ding X, Luo L, Hao Q, Dong B. Disability associated with obesity, dynapenia and dynapenic-obesity in Chinese older adults. J Am Med Dir Assoc. 2014;15(2). doi: 10.1016/j.jamda.2013.10.009 [Crossref]
- Bektas A, Schurman SH, Sen R, Ferrucci L. Aging, inflammation and the environment. Exp Gerontol. 2018;105:10-18. doi: 10.1016/j.exger.2017.12.015 [Crossref]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(Suppl1):S4-9. doi: 10.1093/gerona/glu057 [Crossref]
- Minciullo PL, Catalano A, Mandraffino G, Casciaro M, Crucitti A, Maltese G, Morabito N, Lasco A, Gangemi S, Basile G. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp (Warsz). 2016;64(2):111-26. doi: 10.1007/s00005-015-0377-3 [Crossref]
- Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. 2017;8:1745. doi: 10.3389/fimmu.2017.01745 [Crossref]
- Tuttle CSL, Thang LAN, Maier AB. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res Rev. 2020;64:101185. doi: 10.1016/j.arr.2020 [Crossref]
- Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021;12(1):330. doi: 10.1038/s41467-020-20123-1 [Crossref]
- Antunes BM, Rossi FE, Inoue DS, Neto JCR, Lira FS. Immunometabolism and exercise: new avenues. Motricidade. 2017;13(1):85-89. doi: 10.6063/motricidade.7941 [Crossref]
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71-82. doi: 10.1001/jama.2012.113905 [Crossref]
- Celis-Morales CA, Welsh P, Lyall DM, Steell L, Petermann F, Anderson J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ. 2018;361:k1651. doi: 10.1136/bmj.k1651 [Crossref]
- Legrand D, Adriaensen W, Vaes B, Matheï C, Wallemacq P, Degryse J. The relationship between grip strength and muscle mass (MM), inflammatory biomarkers and physical performance in community-dwelling very old persons. Arch Gerontol Geriatr. 2013;57(3):345-51. doi: 10.1016/j.archger.2013.06.003 [Crossref]
- Paranhos Amorim DN, Nascimento DDC, Stone W, Alves VP, Coelho Vilaça E Silva KH. Body composition and functional performance of older adults. Osteoporos Sarcopenia. 2022;8(2):86-91. doi: 10.1016/j.afos.2022.04.002 [Crossref]
- Gadelha AB, Neri SGR, Vainshelboim B, Ferreira AP, Lima RM. Dynapenic abdominal obesity and the incidence of falls in older women: a prospective study. Aging Clin Exp Res. 2020;32(7):1263-70. doi: 10.1007/s40520-019-01318-z [Crossref]
- Rossi AP, Bianchi L, Volpato S, Bandinelli S, Guralnik J, Zamboni M, et al. Dynapenic Abdominal Obesity as a Predictor of Worsening Disability, Hospitalization, and Mortality in Older Adults: Results From the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2017;72(8):1098-104. doi: 10.1093/gerona/glw203 [Crossref]
- Ribeiro JC, Duarte JG, Gomes GAO, Costa-Guarisco LP, de Jesus ITM, Nascimento CMC, et al. Associations between inflammatory markers and muscle strength in older adults according to the presence or absence of obesity. Exp Gerontol. 2021;151:111409. doi: 10.1016/j.exger.2021.111409 [Crossref]
- Corrêa HL, Rosa TDS, Dutra MT, Sales MM, Noll M, Deus LA, et al. Association between dynapenic abdominal obesity and inflammatory profile in diabetic older community-dwelling patients with end-stage renal disease. Exp Gerontol. 2021;146:111243. doi: 10.1016/j.exger.2021.111243 [Crossref]
- Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423-9. doi: 10.1093/ageing/afr051 [Crossref]
- Hiol AN, von Hurst PR, Conlon CA, Mugridge O, Beck KL. Body composition associations with muscle strength in older adults living in Auckland, New Zealand. PLoS One. 2021;16(5):e0250439. doi: 10.1371/journal.pone.0250439 [Crossref]
- Murai J, Nishizawa H, Otsuka A, Fukuda S, Tanaka Y, Nagao H, et al. Low muscle quality in Japanese type 2 diabetic patients with visceral fat accumulation. Cardiovasc Diabetol. 2018;17(1):112. doi: 10.1186/s12933-018-0755-3 [Crossref]
- Barbat-Artigas S, Rolland Y, Cesari M, Abellan van Kan G, Vellas B, Aubertin-Leheudre M. Clinical relevance of different muscle strength indexes and functional impairment in women aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2013;68(7):811-9. doi: 10.1093/gerona/gls254 [Crossref]
- Nascimento DDC, Prestes J, de Sousa Diniz J, Beal PR, Alves VP, Stone W, et al. Comparison of field- and laboratory-based estimates of muscle quality index between octogenarians and young older adults: an observational study. J Exerc Rehabil. 2020;16(5):458-66. doi: 10.12965/jer.2040668.334 [Crossref]
- Melo GLR, Moraes MR, Nascimento EF, Boato EM, Beal FLR, Stone W, et al. Field-based versus laboratory-based estimates of muscle quality index in adolescents with and without Down syndrome. J Intellect Disabil Res. 2022;66(12):1000-8. doi: 10.1111/jir.12959 [Crossref]
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. doi: 10.1093/ageing/afy169 [Crossref]
- Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513-37. doi: 10.1038/s41574-018-0062-9 [Crossref]
- Nakano MM, Otonari TS, Takara KS, Carmo CM, Tanaka C. Physical performance, balance, mobility, and muscle strength decline at different rates in elderly people. J Phys Ther Sci. 2014;26(4):583-6. doi: 10.1589/jpts.26.583 [Crossref]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. J Behavior Research Methods. 2007;39(2):175-91. doi: 10.3758/bf03193146 [Crossref]
- Santos Morais Junior G, Ignacio Valenzuela Perez D, Cecília Tonet-Furioso A, Gomes L, Coelho Vilaça KH, Paulo Alves V, et al. Circulating interleukin-6 (but not other immune mediators) associates with criteria for fried's frailty among very old adults. J Aging Res. 2020;2020:6831791. doi: 10.1155/2020/6831791 [Crossref]
- Valenzuela PL, Maffiuletti NA, Tringali G, De Col A, Sartorio A. Obesity-associated poor muscle quality: prevalence and association with age, sex, and body mass index. BMC Musculoskelet Disord. 2020;21(1):200. doi: 10.1186/s12891-020-03228-y [Crossref]
- Fragala MS, Kenny AM, Kuchel GA. Muscle quality in aging: a multi-dimensional approach to muscle functioning with applications for treatment. Sports Med. 2015;45(5):641-58. doi: 10.1007/s40279-015-0305-z [Crossref]
- Santos EPRD, Silva CFR, Ohara DG, Matos AP, Pinto ACPN, Pegorari MS. Short Physical Performance Battery (SPPB) score as a discriminator of dynapenic abdominal obesity among community-dwelling older adults. Geriatr Nurs. 2021;42(2):467-72. doi: 10.1016/j.gerinurse.2021.02.016 [Crossref]
