Rev Bras Fisiol Exerc. 2023;22:e225513
doi: 10.33233/rbfex.v22i1.5513
REVIEW
Polycystic ovary syndrome: relation with diet and
exercise - systematic review of randomized clinical trials
Síndrome
dos ovários policísticos: relação com dieta e exercício - revisão sistemática
de ensaios clínicos randomizados
Marina
Amorim Santos1, Nathália Rocha Rios Mendes Machado1,
Paulo Gabriel Barbosa de Carvalho1, Márcia Farias Moreira1,
Francisco Paulo Cerqueira Mota1
1Centro Universitário UniFTC,
Salvador, BA, Brazil
Received: May 4, 2023; Accepted: July 18, 2023.
Correspondence: Marina Amorim Santos,
marinaamorimsantos@hotmail.com
How to cite
Santos MA, Machado
NRRM, Carvalho PGB, Moreira MF, Mota FPC. Polycystic ovary syndrome: relation with diet and exercise – systematic
review of randomized clinical trials. Ver Bras Fisiol
Exerc. 2023;22:e225513. doi:
10.33233/rbfex.v22i1.5513
Abstract
Introduction: Women with
polycystic ovary syndrome tend to present overweight/obese and have worst
results for reproduction. Knowing that the effects of weight loss have a
positive factor in the prognosis of fertility, the summary of published
articles about the changes of lifestyle modification has on ovulation/pregnancy
is important for health professionals to reinforce the method as a first-line
therapeutic strategy. Aim: This study aimed to describe the alterations
of lifestyle modifications on ovulation/pregnancy in overweight/obese women
with polycystic ovary syndrome. Methods: It is a systematic review of
randomized clinical trials based on the Preferred Reporting Items guideline for
Systematic Review and Meta-analyses (PRISMA). Publications between 2010-2021 in
Portuguese or English in databases: Pubmed, Scielo, and Lilacs were included. The interest variables
analyzed were: age, body mass index, lifestyle modification, comorbidities, and
reproductive outcome (ovulation/pregnancy). Results: 15 studies were
screened through the databases, and four were manually accessed through the
reference list of the selected articles. After analyzing the eligibility
criteria, five studies were considered relevant and included in this review,
all randomized clinical trials. Conclusion: The lifestyle modification
treatment implementation of overweight/obese women with polycystic ovary
syndrome improved ovulation rate and menstrual regularity.
Keywords: obesity; infertility; healthy
lifestyle.
Resumo
Introdução: Mulheres portadoras da Síndrome dos
Ovários Policísticos apresentam maior tendência a sobrepeso/obesidade e piores
resultados no desfecho reprodutivo. Sabendo que os efeitos da perda de peso
possuem fator positivo no prognóstico da fertilidade, a síntese de estudos
sobre a mudança no estilo de vida na ovulação é importante para que os
profissionais de saúde a reforcem como estratégia terapêutica de primeira
linha. Objetivo: O presente estudo teve como objetivo descrever as
alterações que a mudança no estilo de vida tem na ovulação/gestação em mulheres
com sobrepeso/obesidade portadoras de síndrome dos ovários policísticos. Métodos:
Trata-se de uma revisão sistemática da literatura de ensaios clínicos
randomizados baseada nos critérios do Preferred Reporting Items guideline for Systematic Review and Meta-analyses (PRISMA). Foram
incluídas publicações entre 2010 e 2021, em português e inglês, presentes nas bases
de dados: Pubmed, Scielo e Lilacs. As variáveis de interesse analisadas foram: idade,
índice de massa corporal, mudança de estilo de vida, comorbidades e desfecho
reprodutivo (ovulação/gestação). Resultados: Foram triados 15 estudos
pela base de dados e 4 foram acessados manualmente através da lista de
referências dos artigos selecionados. Após análise dos critérios de
elegibilidade, 5 ensaios clínicos randomizados foram considerados relevantes e
incluídos nesta revisão. Conclusão: A implementação da mudança do estilo
de vida no tratamento de mulheres com sobrepeso/obesidade portadoras de
síndrome dos ovários policísticos demonstrou resultados satisfatórios no
desfecho ovulação, com melhora das taxas e regularidade menstrual.
Palavras-chave: obesidade; infertilidade; estilo de
vida saudável.
Introduction
Polycystic ovary syndrome (PCOS) is
a common endocrine disorder that affects about 10% of women of reproductive age
worldwide [1]. Patients with this condition have a heterogeneous disorder [2]
characterized by dysfunction in the neuro-endocrine-reproductive axis, which
leads to ovarian morphological alteration and high androgen production [3],
which predisposes to the development of metabolic and non-metabolic systemic
disorders that go far beyond infertility.
Women with PCOS who manage to
become pregnant, for example, have a high rate of early miscarriage (30 to 50%)
compared to the average rate in the general population, which is 15% [4]. In
addition, they are at increased risk of pregnancy complications, including
gestational diabetes and hypertensive disorders, which ultimately result in
preterm labor and perinatal mortality [5,6].
Among the causes of infertility and
gestational complications related to PCOS, it is known that pre-gestational
overweight and obesity are relevant as aggravating negative factors, with a
higher body mass index (BMI) being associated with a worse fertility prognosis,
regardless of the mode of conception [5,6,7]. On the other hand, it has been
shown that weight loss improves reproduction and metabolism rates [8,9,10]. Thus,
lifestyle modification (LSM), i.e., a healthy diet and physical activity, has
been recommended as a first-line strategy before any intervention for women
with PCOS and reproductive desire [11,12,13].
Although several studies address
different therapeutic options for patients with PCOS, a comparison between the
already available studies that discuss the implementation of LSM as a
therapeutic strategy is important for the synthesis of the most current
information on its impact on ovulation/gestation. This facilitates the access
of health professionals to this topic, who, in addition to detecting,
preventing, and treating risk factors at an early stage in overweight/obese
patients with PCOS, will be more able to instruct them about the practice of
exercises daily physical exercises and food re-education.
Therefore, this study aimed to
describe the changes that lifestyle modifications have on ovulation/gestation
in overweight/obese patients with polycystic ovary syndrome.
Methods
This study is a systematic review
of the literature based on the criteria of the Preferred Reporting Items
guideline for Systematic Review and Meta-analyses (PRISMA) [14] and registered
in PROSPERO under id: CRD42023421853. To this end, original articles were
selected, including randomized clinical trials published between 2010 and 2021,
in Portuguese and English, found registered in the following databases: Pubmed, Scielo, and Lilacs, which
gathered the available evidence on the alterations that lifestyle modification
has on ovulation/pregnancy in overweight/obese patients with polycystic ovary
syndrome.
Studies whose intervention method
was only drugs that induce ovulation, as well as studies focused exclusively on
obesity and infertility, were excluded. Conference abstracts and unpublished
manuscripts were considered ineligible.
Sample eligibility criteria
Women of reproductive age, aged
between 18 and 40 years, with a BMI ≥ 25 kg/m2, infertile due
to PCOS, submitted to programs composed of exercises and/or diet, were included
in the sample, and no specific definition of exercise or diet was used in the
search strategy to include as many studies as possible.
Study search and selection strategy
The search strategy included the
descriptors and the respective terms translated into Portuguese found in DeCS/MeSH: Polycystic Ovary
Syndrome, Obesity, Ovulation, Pregnancy, Exercise and Diet, and Healthy, which
were used together through Boolean operators (AND) to identify the appropriate
articles.
Other articles that met the
eligibility criteria were manually searched through the reference list of
eligible studies.
Data summary
The studies were sorted by title by
the authors and were analyzed by abstract to see if they met the inclusion
criteria. Duplicate articles were removed manually. Those that did not meet the
criteria were excluded, and the approved ones were analyzed in full for data
extraction that sought information about the sample, intervention protocol
(diet and/or physical exercise), and measurement of ovulation and pregnancy,
including pregnancy complications. All authors participated in the stage, and
disagreements about the selection and/or extraction of data were discussed
among them.
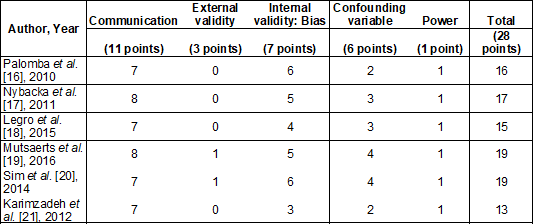
Quality of evidence and risk of bias
The risk of bias in each study was
ascertained using the Downs and Black Risk of Bias tool [15]. It was evaluated
by all the authors. And, in case of disagreement, the finding of the majority
prevailed. This tool is an accurate checklist that provides an overall score
for study quality and is appropriate for evaluating randomized and
non-randomized studies.
Results
Nineteen studies were identified
according to the proposed methodological strategy. After applying the
eligibility criteria, five articles were included in this review, all
randomized clinical trials. Figure 1 shows the selection of articles.
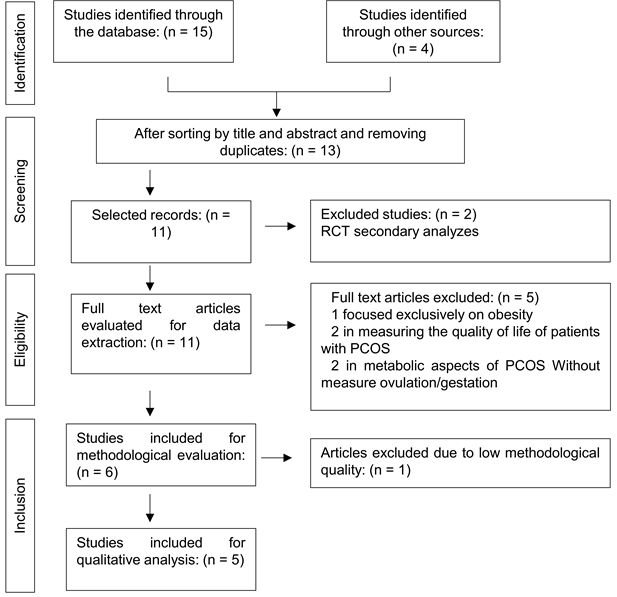
RCT = randomized controlled trial;
PCOS = Polycystic Ovary Syndrome
Figure 1 – Flowchart of article
selection
The samples of the selected studies
ranged from 49 to 577 participants, totaling 928 individuals. The target
population studied was overweight/obese infertile women with PCOS, BMI ≥
25 kg/m2, aged 18-40 years. Regarding the intervention, although all
the articles used the LSM through physical activity and/or diet, the dietary
and exercise strategy used differed between the studies, each one presenting
particularities. This information is more detailed in Table I.
Table I - Qualitative synthesis of
selected articles
Chart 1 – Quality of evidence according
to the Downs and Black scale [15]
Discussion
This review showed that the
lifestyle modifications implemented in the treatment of women with PCOS
significantly improved the evaluated ovulatory patterns, especially when
associated with drug treatment. Soon, it was found that the ovulation rate and
the regularity of menstrual cycles were higher when the intervention had LSM
involved.
It appears that ovulation is
influenced by obesity due to a hormonal imbalance, the main one being insulin
resistance [22]. Previous studies have already proven the relationship between
insulin signaling and steroidogenesis through therapeutic interventions with
insulin sensitizers, which, when implemented, reduced total and free androgen
concentrations and increased SHBG (sex hormone-binding globulin) [8].
Interestingly, these same changes
were observed with weight loss [23] and physical activity practice, as this is
effective in restoring ovulation, regardless of the decrease in BMI [24]. This
happens because exercise alone can modulate inflammatory proteins with a
negative effect on the insulin signal even when it does not cause weight change
[25].
Thus, submitting patients with
PCOS, who tend to accumulate visceral adipose tissue for reasons that are still
unclear, to a routine of physical exercises and a healthy diet allows the
restoration of the hormonal pattern and, consequently, the improvement of the
reproductive outcome. In this regard, the studies' results reviewed here are
consistent with the literature published.
As for the percentage of clinical
pregnancy detected, the studies evaluated did not report relevant differences
concerning the conception rate between the observed groups, except for Sim et
al. [20], who demonstrated a higher pregnancy rate in the group submitted
to lifestyle changes. Furthermore, in terms of gestational complications,
considerable differences between the analyzed groups were also not observed in
most studies, showing that, at least in these cases, LSM has little or no
significant impact.
According to the existing
literature, unfavorable gestational outcomes are distinguished according to the
characteristics of PCOS [26] and occur independently of obesity [27]. Thus,
during pregnancy, there is an increase in adverse outcomes in women with PCOS
compared to women without this syndrome, with the most cited being gestational
diabetes mellitus, preeclampsia, premature birth, and poor perinatal outcomes
[26,27]. Similar results, however, were not found in the present study.
Regarding the limitations of this
review, it is worth mentioning the non-standardization of the interventions
carried out since, in an attempt to include a higher number of studies, no
specific definition of LSM was used. Furthermore, the selected studies were
performed with a relatively small sample of patients. It is also important to
highlight that in this present study, as well as in the scientific evidence
available on this subject, the mechanisms by which physical activity and diet
influence the reproductive outcome in PCOS were not fully clarified. As a
result, it is necessary to continue research on this subject.
The highlight of this review is due
to the most recent information regarding lifestyle changes and PCOS that were
condensed in this study and the scarcity of articles focused on this topic, in
addition to the selectivity of the bibliographic research carried out,
including only randomized clinical trials to increase the level of scientific
evidence.
Conclusion
The implementation of lifestyle
modifications in the treatment of overweight/obese women with PCOS has shown
satisfactory results in the ovulation outcome, with improved rates and
menstrual regularity.
Academic affiliation
This article represents the course
completion work of Marina Amorim Santos, Nathália
Rocha Rios Mendes Machado, Paulo Gabriel Barbosa de Carvalho and Márcia Farias Moreira, supervised by Professor Francisco
Paulo Cerqueira Mota at UniFTC University center, Medical School, Salvador/BA.
Potential conflict of
interest
No conflicts of interest
potentially relevant to this article were reported.
Financing source
There was no external funding
source for this study.
Authors' contribution
Conception and design
of the research: Santos MA, Machado NRR, Carvalho
PGB, Moreira MF; Data analysis and interpretation: Santos MA, Machado
NRR, Carvalho PGB e Moreira MF; Writing of the manuscript: Santos MA,
Machado NRR, Carvalho PGB e Moreira MF; Critical review of the manuscript
for important intellectual content: Santos MA, Machado NRR, Carvalho PGB e
Moreira MF.
References
- Cooney LG, Dokras A. Beyond
fertility: polycystic ovary syndrome and long-term health. Fertil
Steril. 2018;110(5):794-809. doi: 10.1016/j.fertnstert.2018.08.021 [Crossref]
- Andrade VHL, Mata AMOF, Borges RS, Silva DRC, Martins
LM, Ferreira PMP, et al. Current aspects of polycystic ovary syndrome: A
literature review. Rev Assoc Med Bras. 2016;62(9):867-871. doi: 10.1590/1806-9282.62.09.867 [Crossref]
- Fernandes
CE, Sá MFS. Tratado de Ginecologia FEBRASGO. 1 ed. São Paulo:
Elsevier; 2018.
- Hoffman BL, Schorge JO,
Bradshaw KD, Halvorson LM, Schaffer JI. Ginecologia
de Willians. 2ª ed. Porto Alegre: Artmed;
2014.
- Joham AE, Palomba S, Hart R. Polycystic ovary syndrome, obesity, and pregnancy. Semin Reprod Med. 2016;34(2):93-101. doi: 10.1055/s-0035-1571195 [Crossref]
- Carneiro
JS, Rosa e Silva AC. Complicações gestacionais e perinatais em mulheres com
síndrome dos ovários policísticos. Femina.
[Internet]. 2021 [cited 2022 Mar 12];49(9):530-6. Available from:
https://www.febrasgo.org.br/media/k2/attachments/FeminaZ2021Z49Z09ZWEBZ1.pdf
- Manual
de ginecologia endócrina. São Paulo: FEBRASGO; 2015 [internet]. Cited 2022 May
12]. Available from: https://mediacdns3.ulife.com.br/PAT/Upload/2005426/disparadorManual_Ginecologia_Endocrina_20200827140253.pdf
- Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020;1;105(8):e2695-709. doi: 10.1210/clinem/dgaa285 [Crossref]
- Azevedo JD, Costa EC, Micussi MTABC, Sá JCF. Modificações do estilo de vida na síndrome dos ovários policísticos: papel do exercício físico e importância da abordagem multidisciplinar. Rev Bras Ginecol Obstet. 2008;30(5):261-67. doi: 10.1590/S0100-72032008000500009 [Crossref]
- Doherty DA, Newnham JP, Bower C, Hart R. Implications of polycystic ovary syndrome for pregnancy and for the health of offspring. Obstet Gynecol 2015;125(6):1397-406. doi: 10.1097/AOG.0000000000000852 [Crossref]
- Campos AE, Leão MEB, Souza MA. O impacto da mudança do estilo de vida em mulheres com síndrome dos ovários policísticos. REAS 2022;13(2):e4354. doi: 10.25248/reas.e4354.2021 [Crossref]
- Colégio
Brasileiro de Medicina do Estilo de Vida [Internet]. Medicina do estilo de Vida
[cited 2022 May 14]. Available from:
https://cbmev.org.br/medicina-do-estilo-de-vida/
- Guia de
Atividade Física para a População Brasileira [Internet]. Ministério da Saúde [cited 2022 May 14]. Available from:
https://bvsms.saude.gov.br/bvs/publicacoes/guia_atividade_fisica_populacao_brasileira.pdf
- Galvão TF, Pansani TSA, Harrad D. Principais itens para relatar revisões sistemáticas e meta-análises: A recomendação PRISMA. Epidemiol Serv Saúde 2015;24(2):335-42. doi: 10.5123/S1679-49742015000200017 [Crossref]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-84. doi: 10.1136/jech.52.6.377 [Crossref]
- Palomba S, Falbo A, Giallauria F, Russo T, Rocca M, Tolino A, Zullo F, Orio F. Six weeks of structured exercise training and hypocaloric diet increases the probability of ovulation after clomiphene citrate in overweight and obese patients with polycystic ovary syndrome: a randomized controlled trial. Hum Reprod. 2010;25(11):2783-91. doi: 10.1093/humrep/deq254 [Crossref]
- Nybacka Å, Carlström K, Ståhle A, Nyrén S, Hellström PM, Hirschberg AL. Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil Steril. 2011;96(6):1508-13. doi: 10.1016/j.fertnstert.2011.09.006 [Crossref]
- Legro RS, Dodson WC, Kunselman AR, Stetter CM, Kris-Etherton PM, Williams NI, et al. Benefit of delayed fertility therapy with preconception weight loss over immediate therapy in obese women with PCOS. J Clin Endocrinol Metab. 2016;101(7):2658-66. doi: 10.1210/jc.2016-1659 [Crossref]
- Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Randomized trial of a lifestyle program in obese infertile women. N Engl J Med. 2016;374(20):1942-53. doi: 10.1056/NEJMoa1505297 [Crossref]
- Sim KA, Dezarnaulds GM, Denyer GS, Skilton MR, Caterson ID. Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: a randomized controlled trial. Clin Obes. 2014;4(2):61-8. doi: 10.1111/cob.12048 [Crossref]
- Karimzadeh MA, Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril. 2010;94(1):216-20. doi: 10.1016/j.fertnstert.2009.02.078 [Crossref]
- Hakimi O, Cameron LC. Effect of exercise on ovulation: a systematic review. Sports Med. 2017;47(8):1555-67. doi: 10.1007/s40279-016-0669-8 [Crossref]
- Kuchenbecker WKH, Groen H, van Asselt SJ, Bolster JHT, Zwerver J, Slart RHJ, et al. In women with polycystic ovary syndrome and obesity, loss of intra-abdominal fat is associated with resumption of ovulation. Hum Reprod. 2011;26:2505-12. doi: 10.1093/humrep/der229 [Crossref]
- Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2011;17(2):171-83. doi: 10.1093/humupd/dmq045 [Crossref]
- Pauli JR, Cintra DE, Souza CT, Ropelle ER. Novos mecanismos pelos quais o exercício físico melhora a resistência à insulina no músculo esquelético. Arq Bras Endocrinol Metabol. 2009;53(4):399-408. doi: 10.1590/S0004-27302009000400003 [Crossref]
- McDonnell R, Hart RJ. Pregnancy-related outcomes for women with polycystic ovary syndrome. Womens Health (Lond). 2017;13(3):89-97. doi: 10.1177/1745505717731971 [Crossref]
- Rees DA, Jenkins-Jones S, Morgan CL. Contemporary reproductive outcomes for patients with polycystic ovary syndrome: a retrospective observational study. J Clin Endocrinol Metab. 2016;101(4):1664-72. doi: 10.1210/jc.2015-2682 [Crossref]
