Rev Bras Fisiol Exerc. 2024;23:e235577
doi: 10.33233/rbfex.v23i2.5577
ORIGINAL ARTICLE
Correlation between stroke severity and functional dependence of
hospitalized patients
Correlação entre a
gravidade do acidente vascular cerebral e a dependência funcional de pacientes
internados
Isadora Oliveira Freitas
Barbosa1, Érika Letícia Gomes Nunes1, Letícia de Souza
Pereira2, Bruna Kelly Ferreira1, Maristela Lúcia Soares
Campos1, Jefferson Petto3,4, Giulliano
Gardenghi1,5,6,7
1Hospital de Urgências de Goiás, Goiânia,
GO, Brazil
2Secretaria Estadual de Saúde, Goiânia,
GO, Brazil
3Escola Bahiana de Medicina e Saúde
Pública, Salvador, BA, Brazil
4Actus-Cordios,
Reabilitação Cardiovascular, Respiratória e Metabólica, Salvador, BA, Brazil
5Hospital ENCORE, Aparecida de Goiânia,
GO, Brazil
6Clínica de Anestesia (CLIANEST),
Goiânia, GO, Brazil
7Faculdade CEAFI,
Goiânia, GO, Brazil
Received March 15,
2024; Accepted on: April 3,
2024.
Correspondence: Isadora Oliveira Freitas Barbosa, isadora.oolvrfisio@gmail.com
How to
cite
Barbosa IOF, Nunes
ELG, Pereira LS, Ferreira BK, Campos MLS, Petto J, Gardenghi
G. Correlation between stroke severity and functional dependence of hospitalized
patients. Rev Bras Fisiol Exerc. 2024;23:e5577. doi:
10.33233/rbfex.v23i2.5577
Abstract
Introduction: Some patients suffering from a stroke have functional
capacity limitations since the hospital stay. The severity of the condition may
be a factor that correlates with the degree of functional dependence of these
individuals. Objective: To analyze the correlation between stroke
severity and the functional status of patients admitted to a reference hospital
in the central-western region of Brazil. Methods: This is an analytical
cross-sectional study, in which the sample was carried out by convenience.
Functional status assessment was performed using the Functional Independence
Measure (FIM) and Barthel scales, while stroke classification was obtained using
the National Institute of Health Stroke Scale (NIHSS). Results: 68
participants were evaluated, with an average age of over 60 years. In the
analyzes between the stroke severity index and the functionality measures
assessed by the FIM and Barthel Index, an inverse correlation was identified (p
< 0.001). 48.5% of patients were
classified as less severe, 42.6% as moderate, 4.4%
as moderate to severe and 4.4% as severe. Regarding the assessment of functionality, evaluated by
the FIM, 5.9% were classified as complete dependence, 32.4% modified dependence
with assistance in up to 50% of activities, 20.6% modified dependence with
assistance in up to 25% of activities. activities and 41.2% with
complete/modified independence. Conclusion: The findings of the present
study point to an unfavorable correlation between the degree of stroke and
functionality, indicating a negative effect of increased severity on the
participants' functionality.
Keywords: stroke; functional status; patient acuity.
Resumo
Introdução: Pacientes acometidos por acidente
vascular cerebral podem possuir limitações da capacidade funcional. A gravidade
do acometimento pode ser um fator que se correlaciona com o grau de dependência
funcional desses indivíduos. Objetivo: Analisar a correlação entre a
gravidade do AVC e o estado funcional de pacientes internados em um hospital de
referência da região centro-oeste do Brasil. Métodos: Trata-se de um
estudo transversal analítico, em que a amostra foi realizada por conveniência.
A avaliação do estado funcional foi realizada através das escalas Medida de
Independência Funcional e Índice de Barthel, enquanto
a classificação do acidente vascular cerebral foi obtida através da National Institute of Health Stroke Scale (NIHSS). Resultados: Foram avaliados 68
participantes, com idade média superior a 60 anos. Nas análises entre a
gravidade do AVC e as medidas de funcionalidade, foi identificada correlação
inversa (p < 0,001). Dentre os participantes, 48,5% foram classificados como
menor gravidade, 42,6% gravidade moderada, 4,4% moderada a grave e 4,4% como
grave. No que se refere a avaliação da funcionalidade, avaliada pela MIF foram
classificados 5,9% como dependência completa, 32,4% dependência modificada com
assistência em até 50% das atividades, 20,6% dependência modificada com
assistência em até 25% das atividades e 41,2% com independência
completa/modificada. Conclusão: Os achados do presente estudo apontam
uma correlação inversa entre o grau de AVC e funcionalidade, indicando efeito
negativo do aumento da gravidade sobre a funcionalidade dos participantes.
Palavras-chave: acidente vascular cerebral; gravidade
do paciente; estado funcional.
Introduction
Among cerebrovascular diseases, stroke has had a major
impact on health, with an increase in the incidence rate in Brazil in recent
years [1]. It is estimated that, each year, approximately 795,000 people suffer
a new or recurrent stroke and that 1 in 4 people over the age of 25 will have a
stroke in their lifetime, making it the second leading cause of death and
morbidity worldwide [2,3].
Stroke is characterized by neurological impairment that
begins suddenly, lasting more than 24 hours, with presumed vascular origin [4].
Functional disability affects a large proportion of these patients and can
include sensory and motor deficits, such as hemiparesis, dysphagia, neglect and
other local neurological deficits, which directly affect the quality of life of
these individuals [2].
Functional improvement after stroke is driven by neural
recovery, neuroplasticity and the individual's physiological and psychosocial
adaptation to functional impairments, which may include aspects of functional
rehabilitation [5].
In the hospital environment, these patients can be
classified according to the severity of the stroke, as well as the level of
functional impairment. To assess the severity of a stroke, the National
Institute of Health Stroke Scale (NIHSS) can be used, which makes it possible
to classify its severity according to the scores obtained [6]. To assess
functional dependence, scales such as the Functional Independence Measure
(FIM), widely used in the hospital environment, and the Barthel Index, known
for evaluating basic activities of daily living in stroke patients, can be used
[7,8].
In view of this, the objective of the present study is to
evaluate the correlation between stroke severity and the functional dependence
of these patients in the hospital phase. Understanding this correlation can be
significant in helping to decide on the best hospital therapeutic plan for
these patients, given the severity of each case.
Methods
This is a cross-sectional, analytical study, carried out
in the wards of a reference emergency and trauma hospital in the Central-West
region of Brazil.
The research was approved by the Research Ethics
Committee (REC), CAAE: 62102322.6.0000.0033. After approval by the REC, a
search was carried out in electronic medical records and patients who matched
the study profile were selected. Data collection was carried out in the
hospital wards by previously trained evaluators, initially applying the
evaluation form with clinical and demographic data and then the evaluation
scales. The assessments were carried out over four months, covering the period
from November 2022 to February 2023.
Individuals over the age of 18, diagnosed with stroke,
who were hospitalized in the hospital wards and who agreed to participate in
the research by signing the Free and Informed Consent Form (ICF) were included.
Patients with previous functional dependence, chronic obstructive pulmonary
disease and/or asthma, and those with a hospital stay of more than 10 days were
excluded. The length of stay was limited so that the NIHSS could be applied.
To determine the degree of stroke, the NIHSS was used,
being a reliable instrument that allows this classification through the
evaluation of 15 items. Severity
can be classified
according to the score, as minor (1 to 4), moderate (5 to 15), moderate to severe (16 to
20) and severe (21 to 42) [6].
The patient's functional status was assessed using the
Barthel and FIM scales. The Barthel Index quantifies functionality through
activities of daily living. The total score ranges from 0 to 100, where 100
indicates greater independence [7].
The FIM assesses functionality based on the performance
of motor and cognitive tasks in activities of daily living. The final score is
calculated by adding the points assigned to each item, which can vary between
18 and 126 points. The level of dependence will be classified according to the
total FIM score, which is: 18: complete dependence; 19 to 60: modified
dependence with assistance in up to 50% of activities; 61 to 103: modified
dependence with assistance in up to 25% of activities; and 104 to 126: complete/modified
independence [8].
The Johns Hopkins scale is used to assess the patient's
mobility over a 12-hour period, or at the time of the professional's approach.
Its score is defined according to the patient's ability to perform the proposed
task, with a score that varies from 1 to 8, with the highest score determining
greater functional mobility [9].
The assessment of muscular strength was carried out using
handgrip strength (HGS), used as a general indicator of muscular strength and
power. To be evaluated, the participant was seated with the spine erect,
holding the dynamometer, where the shoulder was placed in a neutral position
and the elbow was flexed at 90º. Maximum isometric grip strength is requested,
3 times on each limb, with a 30-second rest interval between repetitions. Only
the best result of each member is considered [10]. The reference values
considered were described in Table I, according to a study developed by Jorge et
al. [11].
Table I - Handgrip strength values, according to Jorge et al.
[11]

HGS = handgrip strength. Kgf: kilogram/force
Scores were calculated and patient classifications were
determined regarding specific assessments in the statistical system Package for
the Social Sciences - SPSS (version 23.0), in addition, all clinical data from
other forms were also transformed into codes and organized in the same program.
The data were analyzed in descriptive terms to characterize the data, and
normal continuous variables were calculated as mean, standard deviation and 95%
confidence interval; continuous variables were not calculated into medians and
interquartile range; categorical variables were analyzed in terms of frequency
and percentage. Spearman correlation was also performed for non-parametric
data. An r value was considered to be between 0.70 - 1 strong correlation, 0.31
- 0.69 moderate correlation and 0 - 0.30 weak correlation. P < 0.05 was
adopted as the level of clinical significance.
Results
70 individuals were included, 2 being excluded due to the
impossibility of completing the evaluations, totaling 68 patients hospitalized
for stroke, 58 (85.3%) ischemic and 10 (14.7%) hemorrhagic, predominantly male
with a mean age greater than 60 years, as shown in Table II.
Table II – Characteristics of included participants
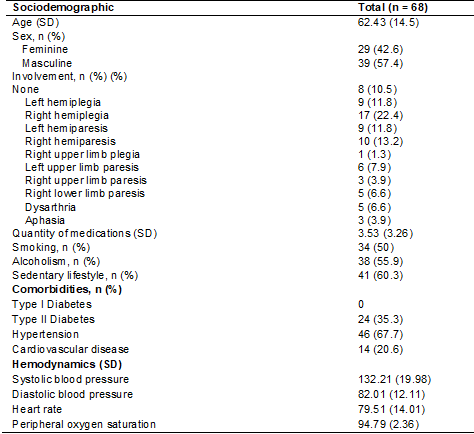
SD =
standard deviation; N = number
Stroke severity assessed using the NIHSS was classified
for 33 (48.5%) as minor, 29 (42.6%) as moderate, 3 (4.4%) as moderate to severe and
3 (4.4%) as serious. The evaluation of
the functional independence measure of the participants categorized 4 (5.9%) as
complete dependence, 22 (32.4%) modified dependence with assistance in up to
50% of activities, 14 (20.6%) modified dependence with assistance in up to 25% of
activities and 28 (41.2%) with complete/modified independence. The mean NIHSS
and MIF scores as well as the results of the other measures collected are
presented in Table III.
Table III – Scoring and classification of participant
assessment measures

SD = standard deviation; NIHSS = National Institute of
Health Stroke Scale; Kgf = kilogram/force; N = number
Regarding the assessment of mobility in bed, 4 (5.9%)
were able to lie down, 7 (10.3%) performed transfers in bed, 16 (23.5%) sat at
the bedside, 7 (10 .3%) performed transfers outside the bed, 2 (2.9%) remained
in an upright position for 1 minute, 5 (7.4%) walked 10 steps or more, 5 (7.4%)
walked 7.5 meters or more a further 22 (32.4%) walked 75 meters or more.
Regarding handgrip strength, when compared with the
healthy population, our results indicate heterogeneous values, within the
average among the population aged 20 to 50 years, and reduced values in the
population over 60 years old, as seen in Table IV.
Table IV - Handgrip strength
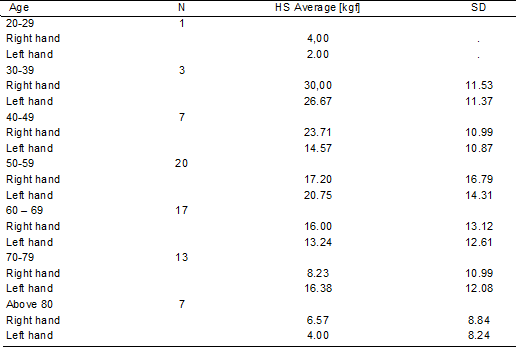
SD = standard deviation; HGS = handgrip strength; Kgf =
kilogram/force; N =number
In the analyzes between the stroke severity index and the
functionality measures assessed by the FIM and the Barthel Index, a large
inverse correlation was identified, indicating a negative effect of increased
severity on the participants' functionality. On the other hand, the increase in
manual strength correlated positively with the assessment of the patient's
mobility, assessed using the Johns Hopkins scale, as seen in Figure 1.
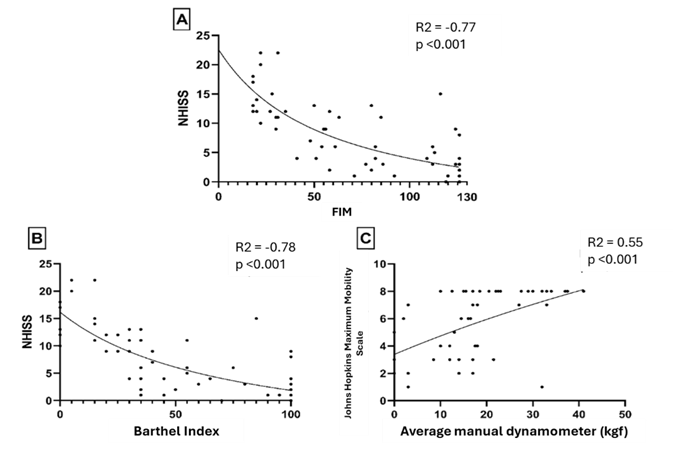
NIHSS = National Institute of Health Stroke Scale; Kgf =
kilogram/force
Figure 1 – Spearman correlation measurement between stroke
severity index and functionality measures (A and B), and manual dynamometry
with hospital mobility scale (C). Stroke: cerebrovascular accident
Discussion
Regarding the main objective of the study, the results
show a negative correlation between the severity of the stroke, assessed by the
NIHSS, and the degree of functionality, assessed by the FIM and Barthel scales.
The data corroborate a study by Jang et al. [12], who point out a better
recovery after hospital discharge in individuals who were classified as having
a lower stroke severity on admission, that is, the lower the severity of the
stroke, the better the functional prognosis after discharge. Brandão et al.
[13] evaluated the correlation between the severity of the stroke and the
degree of dysphagia, evaluated as functionality parameters, observing a
negative correlation, which consolidates the results of the present study.
The NIHSS scale is widely used to classify stroke
severity, being well disseminated in the literature and identified as a
predictor of mortality, functionality and outcome [14,15,16]. Research conducted
by Bhaskar et al. [14] evaluated the correlation between stroke severity
and functionality, using, respectively, the NIHSS and the modified Rankin
scale. Consolidating the data found in this study, they concluded that there
was a moderate positive correlation between the NIHSS scores on admission and
the Rankin scale score 90 days after admission.
Regarding the level of functionality of the sample
studied, our research showed that 19.1% of patients were completely dependent;
26.5, severe dependence; 11.8, moderate dependence; 14.7, mild dependence; and
27.9, total independence. The study conducted by Baskhar et al. [14]
showed that 61% of patients reported unfavorable functional results at
discharge and 66% evaluated 3 months after the onset of the stroke. The data
are compatible with the findings of our research, since only 27.9% of the
patients evaluated showed total functional independence.
Regarding the assessment of handgrip strength, it was
heterogeneous in the population aged 20 to 59 years and reduced in individuals
over 60 years of age. Regarding the assessment of mobility in bed, 4 (5.9%)
were able to lie down, 7 (10.3%) performed transfers in bed, 16 (23.5%) sat at
the bedside, 7 (10.3%) performed transfers outside the bed, 2 (2.9%) remained
in an upright position for 1 minute, 5 (7.4%) walked 10 steps or more, 5 (7.4%)
walked 7.5 meters or more 22 (32.4%) walked 75 meters or more. When analyzing
the data above, it was observed that 60.3% of patients were able to carry out
their activities outside of bed, which can be explained by the intervention of
physiotherapy while still in the hospital environment.
Li et al. [17] carried out a meta-analysis that
aimed to research the effect of early mobilization in patients with stroke in
the acute phase and concluded that early intervention did not change the Rankin
scale score but was associated with better Barthel scale scores. Bernhardt et
al. [18] pointed out that early intervention seems to improve the quality
of life in patients in intensive care, which highlights that hospitalized
patients have significant benefits if mobilized early. A systematic review with
meta-analysis conducted by Miranda et al. [19] recently concluded that
early mobilization should be started 24 hours after the stroke, with short-term
exercises aimed at sitting, standing up and walking, which emphasizes the
relevance of stimulating functionality in the early stages.
Our research showed that there is a moderate positive
correlation between the degree of hospital mobility and handgrip strength,
which can be explained by the correlation described between handgrip strength
and global muscle strength [20]. The results highlight the relevance of
implementing training in the hospital phase, to contribute to a better
functional prognosis.
Studies indicate that early
mobilization improves the prognosis of stroke patients after hospitalization,
as it encourages transfers, position changes, sitting and early ambulation of
patients [18,21,22]. Although it is known that the capacity for neuroplasticity
is influenced by the initial nature of the injury, it is difficult to draw a
functional prognosis for these patients [23].
Regarding the characterization of the sample in the
present study, the majority were male (57.4%), with an average age of over 60
years, which is similar to the population of other studies referring to stroke
[14,24]. Brandão et al. [13] showed that, although the prevalence of
stroke is higher in males, female gender and age are associated with a worse
functional prognosis and higher mortality.
Regarding the lifestyle habits evaluated in this
research, 50% of patients reported smoking. A systematic review with
meta-analysis, conducted by Pan et al. [25], showed that smoking
patients have an overall increased risk of stroke compared to non-smokers, with
a higher risk for current smokers compared to former smokers. Smoking is
associated with both pulmonary and cardiovascular diseases, which poses more
risks to these patients, as this association can lead to worse outcomes [26].
Regarding the consumption of alcoholic beverages, more
than half of the participants (55%) reported drinking alcohol. Studies indicate
that its excessive consumption is directly associated with cardiovascular
diseases, causing important changes in blood pressure [27,28]. A meta-analysis
of prospective studies, conducted by Zhang et al. [29], investigated the
dose-response relationship of alcohol consumption with stroke. It pointed out
that low alcohol intake is related to a lower risk of morbidity and mortality,
while excessive alcohol intake is associated with increased risks in these
patients.
Among the comorbidities found in the present study, the
majority of individuals reported having high blood pressure (67.7%), followed
by type II diabetes (35.3%) and cardiovascular diseases (20.6%). Arterial
hypertension is the most important modifiable risk factor for stroke [32]. The
study conducted by Sokol et al. [33] showed that reducing systolic blood
pressure by 2 mmHg was associated with a 25% reduction in the risk of stroke,
while reducing diastolic blood pressure was associated with a 50% lower risk,
highlighting the need to maintain adequate blood pressure control. arterial.
In relation to heart diseases, the literature points to
an important correlation with stroke, with atrial fibrillation (AF) being one
of the main causes, since the displacement of the thrombus formed in the
cardiac vessels impacts distally on brain regions, causing stroke. In addition
to AF, other heart diseases are identified as factors associated with the
disease, such as atrial septal aneurysm, patent foramen ovale, cardiomyopathy,
left ventricular dysfunction, infective endocarditis and aortic atheromatosis
[34]. Among the participants in our study, 20.6% reported having heart disease,
but these were not specified due to the participants' lack of knowledge.
Another factor reported in our research was the presence
of type 2 diabetes, which is associated with a higher risk of cardiovascular
events [35]. The study by Barbaresko et al. [36] showed that patients
with type 2 diabetes are associated with the risk of stroke and myocardial
infarction. Hill [35] pointed out that diabetic patients are approximately
twice as likely to have a stroke compared to non-diabetics in any age group.
The study carried out has important limitations regarding
the number of participants who made up the sample. The research was restricted
to a period of four months, having a limited number of participants. We suggest
that new studies be carried out that can cover a larger sample.
Conclusion
The findings of the present study point to an unfavorable
correlation between the degree of stroke and functionality, indicating a
negative effect of increased severity on the participants' functionality.
Based on the data highlighted above, it can be inferred
that it is essential to measure the severity of the stroke to estimate a
prognosis related to functional capacity and mortality risk. Furthermore, the
importance of presenting the measured score in the medical record must be
highlighted, so that rehabilitation professionals can categorize care and
program a treatment plan based on each patient's prognosis. We also emphasize
that early mobilization should be part of the treatment plan, as it is associated
with greater functional benefits.
Conflicts of interest
The authors have no conflict of interest in publishing
this article.
Sources of financing
None
Authors' contributions
Conception and design of the research: Nunes ELG, Gardenghi G; Data collection: Barbosa IOF, Ferreira BK,
Campos MLS; Data analysis and interpretation: Barbosa IOF, Nunes ELG,
Pereira LS, Gardenghi G; Manuscript writing: Barbosa IOF, Nunes ELG,
Gardenghi G; Critical review of the manuscript: Barbosa IOF, Nunes ELG,
Pereira LS, Ferreira BK, Campos MLS, Petto J, Gardenghi G.
References
- Margarido AJL, Gomes AFSR,
Araújo GLS, Pinheiro CM, Barreto LB. Epidemiologia do acidente vascular
encefálico no Brasil. Revista Eletrônica Acervo Científico. 2021;39:8859. doi: 10.25248/reac.e8859.2021 [Crossref]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M,
Das SR, Deo R, et al. Heart Disease and
Stroke Statistics—2017 Update: a report from the American Heart Association.
Circulation. 2017;135(10). doi: 10.1161/cir.0000000000000485 [Crossref]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al.
Heart Disease and Stroke Statistics—2016 Update. Circulation. 2016;133(4). doi: 10.1161/cir.0000000000000350 [Crossref]
- Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, et al. The
World Health Organization STEPwise approach to noncommunicable disease
risk-factor surveillance: methods, challenges, and opportunities. Am J Public
Health. 2016;106(1):74-8. doi: 10.2105/ajph.2015.302962 [Crossref]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, et al.
Harnessing neuroplasticity for clinical applications. Brain.
2011;134(6):1591-609. doi: 10.1093/brain/awr039 [Crossref]
- Fonarow GC, Saver JL, Smith EE, Broderick JP, Kleindorfer DO, Sacco RL, et
al. Relationship of National Institutes of Health Stroke Scale to 30-day
mortality in medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc. 2012;1(111):42-50. doi: 10.1161/xjaha.111.000034 [Crossref]
- Minosso JS, Amendola F, Alvarenga MR, Oliveira
MA. Validação, no Brasil, do Índice de Barthel em
idosos atendidos em ambulatórios. Acta Paul Enferm. Abr 2010; 23(2):218-23. doi: 10.1590/s0103-21002010000200011 [Crossref]
- Riberto M, Miyazaki MH,
Jorge Filho D, Sakamoto H, Battistella LR. Reprodutibilidade da versão
brasileira da Medida de Independência Funcional. Acta Fisiatr.
2001;8(1):45-52. doi: 10.5935/0104-7795.20010002 [Crossref]
- Hoyer EH, Young DL, Klein LM, Kreif J, Shumock K, Hiser S, et al. Toward a
common language for measuring patient mobility in the hospital: reliability and
construct validity of interprofessional mobility measures. Phys Ther.
2017;98(2):133-42. doi: 10.1093/ptj/pzx110 [Crossref]
- Jeong M, Kang HK, Song P, Park HK, Jung H, Lee SS, et al. Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Chronic Obstr Pulm Dis. 2017;12:2385-90. doi: 10.2147/copd.s140915 [Crossref]
- Jorge MSG, Ribeiro DS,
Garbin K, Moreira I, Rodigheri PV, Lima WG et al.
Valores de la fuerza de prensión palmar en una población de diferentes edades. Lecturas:
Educación Física y Deportes [Internet]. 2019 [cited 2023 oct 12];23(249):56-69.
Available from:
https://efdeportes.com/efdeportes/index.php/EFDeportes/article/view/296
- Jang MU, Kang J, Kim BJ, Hong JH, Yeo MJ, Han MK, et al. In-hospital and
post-discharge recovery after acute ischemic stroke: a Nationwide Multicenter
Stroke Registry-base Study. J
Korean Med Sci. 2019;34(36):1-12. doi: 10.3346/jkms.2019.34.e240 [Crossref]
- Brandão BC, Silva MA,
Rodrigues CG, Damando MD, Lourenção LG. Relação entre
ingestão oral e gravidade do Acidente Vascular Cerebral Agudo. CoDAS. 2020;32(5):1-6. doi: 10.1590/2317-1782/20202018154 [Crossref]
- Bhaskar S, Stanwell P, Bivard A, Spratt N, Walker R, Kitsos G, et al. The influence of initial stroke severity on mortality, overall functional outcome and in-hospital placement at 90 days following acute ischemic stroke: A tertiary hospital stroke register study. Neurol India. 2017;65(6):1252-59. doi: 10.4103/0028-3886.217947 [Crossref]
- Tseng MC, Chang KC. Stroke
severity and early recovery after first-ever ischemic stroke: Results of a hospital-based study in Taiwan. Health Policy. 2006;79(1):73-8. doi: 10.1016/j.healthpol.2005.12.003 [Crossref]
- Sakthivadivel V, Ramachandran
K, Radha D, Gaur A, Kaliappan A. Is the National Institute
of Health Stroke Scale a valid prognosticator
of the aftermath
in patients with ischemic stroke? J Fam Med Prim Care.
2022;11(11):7185-90. doi: 10.4103/jfmpc.jfmpc_611_22 [Crossref]
- Li Z, Zhang X, Wang K, Wen
J. Effects of early mobilization after acute stroke:
a meta-analysis of randomized control trials. J Stroke Cerebrovasc Dis.
2018;27(5):1326-37. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.021 [Crossref]
- Bernhardt J, English C, Johnson L, Cumming TB.
Early mobilization after stroke. Stroke. 2015;46(4):1141-6. doi: 10.1161/strokeaha.114.007434 [Crossref]
- Miranda JMA, Borges VM, Luvizutto GJ, Shinosaki JSM. Early mobilization in
acute stroke phase: a systematic review. Top Stroke Rehabil.
2021;30(2):157-168. doi: 10.1080/10749357.2021.2008595 [Crossref]
- Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation
in grip strength: a systematic review and meta-analysis of normative data. Age
Ageing. 2016;45(2):209-16. doi: 10.1093/ageing/afv192 [Crossref]
- Diserens K, Moreira T, Hirt L, Faouzi M, Grujic J, Bieler G, et al. Early
mobilization out of bed after ischaemic stroke reduces severe complications but
not cerebral blood flow: a randomized controlled pilot trial. Clin Rehabil.
2011;26(5):451-9. doi: 10.1177/0269215511425541 [Crossref]
- Herisson F, Godard S, Volteau C, Le Blanc E, Guillon B, Gaudron M. Early Sitting in Ischemic Stroke Patients (SEVEL): a randomized controlled trial. PlosOne. 2016;11(3):1-13. doi: 10.1371/journal.pone.0149466 [Crossref]
- Prabhakaran S, Zarahn E, Riley C, Speizer A, Chong JY, Lazar RM, et al.
Inter-individual variability in the capacity for motor recovery after ischemic
stroke. Neurorehabilit Neural Repair. 2007;22(1):64-71. doi: 10.1177/1545968307305302 [Crossref]
- Einstad MS, Saltvedt I, Lydersen S, Ursin MH, Munthe-Kaas R, Ihle-Hansen H,
et al. Associations between post-stroke motor and cognitive function: a
cross-sectional study. BMC Geriatr. 2021;21(1):1-10. doi: 10.1186/s12877-021-02055-7 [Crossref]
- Pan B, Jin X, Jun L, Qiu S, Zheng Q, Pan M. The relationship between
smoking and stroke. Medicine. 2019;98(12):1-8. doi: 10.1097/md.0000000000014872 [Crossref]
- Fischer F, Kraemer A. Meta-analysis of the association between second-hand
smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public
Health. 2015;15(1):1-18. doi: 10.1186/s12889-015-2489-4 [Crossref]
- Piano MR. Alcohol’s effects on the cardiovascular system. Alcohol research:
current reviews [Internet].2017 [citado 2023 out];38(2):219–41. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513687/?report=classic
- Briasoulis A, Agarwal V,
Messerli FH. Alcohol consumption and the risk of
hypertension in men and women: A Systematic Review and Meta-Analysis. J Clin
Hypertens. 2012;14(11):792-8. doi: 10.1111/jch.12008 [Crossref]
- Zhang W, Pan H, Zong Y, Wang J, Xie Q. Respiratory muscle training reduces
respiratory complications and improves swallowing function after stroke: A
systematic review and meta-analysis. Arch Phys Med Rehabil.
2021;103(6):1179-97. doi: 10.1016/j.apmr.2021.10.020 [Crossref]
- Zhuang Z, Gao M, Yang R, Li N, Liu Z, Cao W, et al. Association of physical
activity, sedentary behaviours and sleep duration with cardiovascular diseases
and lipid profiles: a Mendelian randomization analysis. Lipids Health Dis.
2020;19(1):1-11. doi: 10.1186/s12944-020-01257-z [Crossref]
- Prior PL, Suskin N. Exercise for stroke prevention. Stroke Vasc Neurol. Jun
2018;3(2):59-68. doi: 10.1136/svn-2018-000155 [Crossref]
- Sarikaya H, Ferro J, Arnold M. Stroke prevention - medical and lifestyle measures. Eur Neurol. 2015;73(3-4):150-7. doi: 10.1159/000367652 [Crossref]
- Sokol S, Kapoor J, Foody J. Blood pressure reduction in the primary and
secondary prevention of stroke. Curr Vasc Pharmacol. 2006;4(2):155-60. doi: 10.2174/157016106776359862 [Crossref]
- Sila CA. Heart diseases and stroke. Curr Neurol Neurosci Rep.
2006;6(1):23-7. doi: 10.1007/s11910-996-0005-z [Crossref]
- Hill MD. Stroke and diabetes mellitus. Handbook of Clinical Neurology.
2014;126:167–74. doi: 10.1016/B978-0-444-53480-4.00012-6 [Crossref]
- Barbaresko J, Rienks J, Nöthlings U. Lifestyle indices and cardiovascular disease risk: a meta-analysis. Am J Prev Med. 2018;55(4):555-64. doi: 10.1016/j.amepre.2018.04.046 [Crossref]
