Rev Bras Fisiol Exerc. 2024;23(2):e235600
doi: 10.33233/rbfex.v23i2.5600ORIGINAL ARTICLE
Effects of a CrossFit® session on redox state markers
Efeitos de uma sessão
de CrossFit® sobre marcadores do estado redox
Brenna Mirelle
Barbosa Bastos, João Henrique Gomes, Ana Mara de Oliveira e Silva, Renata
Rebello Mendes
Universidade Federal de
Sergipe, Aracaju, SE, Brasil
Recebido em: 6 de julho de
2024; Aceito em: 30 de agosto de 2024.
Correspondência: Renata Rebello Mendes, remendes@academico.ufs.br
How to cite
Bastos BMB, Gomes JH, Oliveira e
Silva AM, Mendes RR. Effects of a CrossFit® session on redox state markers.
Rev Bras Fisiol Exerc 2024;23(2):e235600. doi: 10.33233/rbfex.v23i2.5600
Abstract
Background: CrossFit® is a type of high-intensity functional
training that may have health benefits. The modality has also been criticized
due to the hypothesis that it could increase the risk of injuries due to
oxidative stress generated by the intensity of the exercises. However, there
are few studies evaluating oxidative stress in its practitioners. Objective:
To evaluate the redox state in trained and non-trained adults, of both sexes,
submitted to a high-intensity protocol named 'Cindy'. Methods: We
evaluated 19 participants of a Crossfit® program, divided into beginners and
experienced, women and men. For characterization, we evaluated body
composition, maximal strength and aerobic capacity. For redox state evaluation,
participants performed Cindy and had blood samples collected at pre-exercise
and 30 minutes post-exercise, through biomarkers such as: SOD, GPx, FRAP and
TBARS. Results: At the post-30 moment, there was a significant increase
of GPx in the general population and, according to gender, this increase was in
women (PRE 40.0 ± 3.9 and POS 46.7 ± 8.1), but not among men (PRE 36.4 ± 8.7
and POS 40.7 ± 5.7); we observed significant reduction of SOD, especially in
novices (PRE 3273.1 ± 414.8 and POS 2378.1 ± 781.9); FRAP increased
significantly (PRE 84.09 ± 20.49 and POS 106.27 ± 28.64), being this phenomenon
observed in both sexes and experience levels; TBARS remained unchanged. Conclusion:
A Cindy session promoted GPx and FRAP increase, SOD reduction and TBARS
maintenance in its practitioners.
Keywords: oxidative stress; circuit-based exercise;
high-intensity interval training
Resumo
Introdução: CrossFit ® é
um tipo de treinamento funcional de alta intensidade que pode trazer benefícios
à saúde. A modalidade também tem sido criticada devido à hipótese de que
poderia elevar risco de lesões decorrentes do estresse oxidativo gerado pela
intensidade dos exercícios. Porém, há poucos estudos avaliando estresse
oxidativo em seus praticantes. Objetivo: Avaliar o estado redox em
adultos treinados e não treinados, de ambos os sexos, submetidos a protocolo de
alta intensidade denominado 'Cindy'. Métodos: Foram avaliados 19
participantes de programa de Crossfit®, divididos em novatos e experientes,
mulheres e homens. Para caracterização, avaliamos composição corporal, força
máxima e capacidade aeróbia. Para avaliação de estado redox, os participantes
realizaram Cindy e tiveram amostras de sangue coletadas nos momentos pré-exercício e 30 minutos pós-exercício, por meio de
biomarcadores como: SOD, GPx, FRAP e TBARS. Resultados:
No momento pós-30, houve aumento significativo de GPx
na população geral e, de acordo o sexo, esse aumento se deu nas mulheres (PRE
40,0 ± 3,9 e POS 46,7±8,1), porém não entre os homens (PRE 36,4 ± 8,7 e POS
40,7 ± 5,7); observamos redução significativa de SOD, especialmente nos novatos
(PRE 3273,1 ± 414,8 e POS 2378,1 ± 781,9); a FRAP aumentou significativamente
(PRE 84,09 ± 20,49 e POS 106,27 ± 28,64), sendo esse fenômeno observado em
ambos os sexos e níveis de experiência; TBARS permaneceram inalterados. Conclusão:
Uma sessão de Cindy promoveu aumento de GPx e FRAP,
redução de SOD e manutenção de TBARS em seus praticantes.
Palavras-chave: estresse oxidativo; exercícios em
circuitos; treinamento intervalado de alta intensidade
Introduction
CrossFit® is a type of high-intensity
functional training consisting
of aerobic and anaerobic stimuli,
performed through fast movements and with
little or no rest between sets [1], which has been
linked to improved cardiovascular, metabolic,
and cognitive health, thus reducing
mortality risks [2,3]. However, this modality
has been the target of scrutiny
due to concerns
related to the high risk of
injuries, hypothetically caused
by the high intensity at which
training sessions are performed,
insufficient rest between exercises, and oxidative stress (OS) [4,5,6,7,8].
Although regular physical
exercise is the only health
behavior associated with a decrease in all-cause mortality in humans [9], studies show that intense physical exercise can generate
OS, characterized by situations in which reactive oxygen species (ROS) outweigh antioxidant compounds [10]. According
to Powers et al. [9], this exercise-induced OS can culminate in
beneficial and harmful outcomes for its practitioners, depending on the amount
of ROS produced.
Muscle force production is affected by OS in a biphasic
manner; an optimal level of ROS is required for muscle fibers to generate 100%
of their maximum isometric force production. However, elevations in ROS
concentrations in muscle fibers, above this “ideal point”, result in a decrease
in the muscle’s ability to generate force [9]. Additionally, moderate
concentrations of ROS produced during exercise have been related to increased
mitochondrial biogenesis, synthesis of oxidative enzymes, and greater activation
of the Mammalian Target of Rapamycin (mTOR), the latter being an important
factor for muscle hypertrophy [7]. However, high concentrations of ROS can
result in significant damage to macromolecules, such as proteins, lipids, and
DNA, which can lead to injuries, which have been so often associated with
CrossFit® [11,12].
The research on OS related to CrossFit® is still vague
and scarce. According to our research, to date, only one study has evaluated OS
in CrossFit® practitioners [6]. In this study, the CrossFit® ‘Cindy’ protocol
was compared to a 20-minute session of high-intensity treadmill running. Plasma
lipid hydroperoxides (LOOH), ferric reducing antioxidant power (FRAP), and
protein carbonyl (PC) were evaluated as biomarkers at four different time
points, and the authors concluded that oxidative stress was similar in both
modalities.
Therefore, it is clear that there is a need for more
studies evaluating OS in CrossFit® practitioners, a modality with a growing
number of practitioners. Thus, the present study aimed to assess the redox
state in adult CrossFit® practitioners undergoing a high-intensity training
protocol and compare the outcomes between both sexes and the level of training
experience in the modality.
Methods
Experimental design
This study comprised a total of five visits. After
approval of the research project by the Research Ethics Committee (no.
3,087,955/2018) and recruitment carried out through the social network of a
CrossFit® box in the city of Aracaju, the first visit happened. On this
occasion, the aim was to clarify the objectives and procedures that would
involve the study to coaches and athletes, as well as to deliver the free and
informed consent form (ICF) for later reading and signing by those who met the
inclusion criteria and who were interested in participating. On the second
visit, after receiving the signed ICF, an anthropometric assessment was carried
out to characterize the participants and familiarize them with the ‘Cindy’
training protocol. On the third visit, as part of the ongoing sample
characterization process, a maximum strength test was conducted (one repetition
maximum - 1RM). On the fourth visit, an aerobic capacity test (Yo-yo test) was
performed, finalizing the characterization of the participants (the
participants were already familiar with the 1RM and yo-yo tests). After 48
hours of rest (no physical training allowed), the fifth visit happened, in
which data collection took place: one hour after consuming a standardized
breakfast, the participants underwent the ‘Cindy’ training protocol and blood
collection before and 30 minutes after exercise (Figure 1).
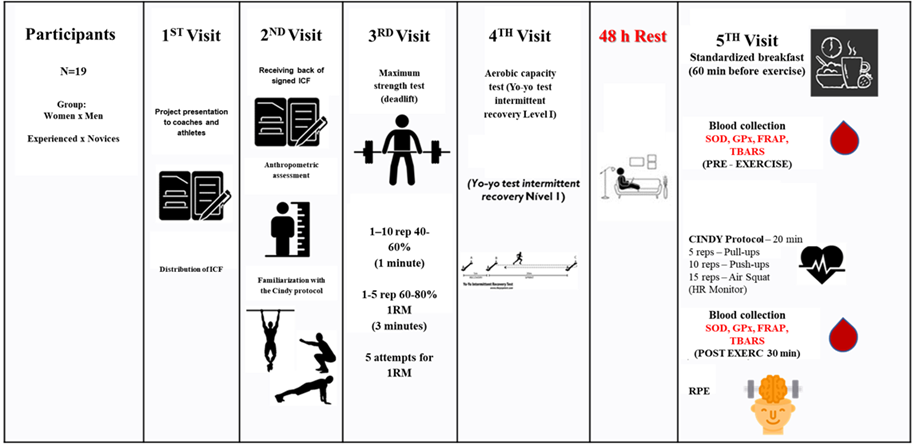
Figure 1 - Experimental design of the study
Participants
Healthy volunteers were recruited from a
CrossFit®-affiliated gym (CrossFit® Quest, Aracaju/SE, Brazil). Inclusion
criteria for participant selection were having at least three months of
experience in a HIFT program and the ability to perform the ‘Cindy’ training
protocol. Novice (NOV) participants were defined as those who had between 3 and
8 months of experience, while experienced (EXP) participants were those who had
more than 18 months of experience (Table I). These time intervals were selected to ensure
a significant gap in experience level between the two groups of participants,
as suggested by Butcher et al. [13].
Although some participants had more than 18 months of
experience, all were classified as recreational CrossFit® practitioners, mainly
because they had never participated in an official competition (except those
organized by the gym). Participants typically performed three to five training
sessions per week.
Participants were excluded if they had: a) between nine
and 17 months of experience; b) any injury or motor disability that prevented
them from performing the tests and training protocol; c) any cardiovascular,
metabolic, or neurological diseases; d) the use of any medication or drugs to
enhance performance; e) the use of supplements containing antioxidant compounds
in the last six weeks, as well as those considered ergogenic [14], such as
caffeine, creatine, beta-alanine, nitrate and bicarbonates, in the last four
months; f) not completing the 20 minutes of the 'Cindy' workout on visit five;
g) not consuming the standardized breakfast before the ‘Cindy’ workout on visit
five.
Table I - Participant characteristics (M ± SD)
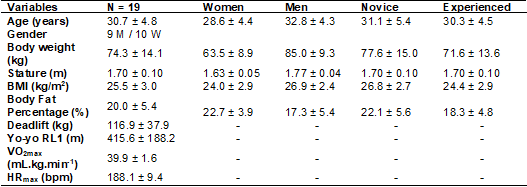
M = Mean; SD = standard deviation; M = men; W = women;
BMI = body mass index; Yo-yo RL1 = yo-yo recovery test level 1; VO2max = maximum
oxygen consumption; HRmax = maximum heart rate
Body composition
The anthropometric assessment covered data such as body
mass, body height, and skin folds, which were later used to calculate the
percentage of fat mass (%FM) and lean mass, according to Jackson and Pollock
[15] and Jackson, Pollock, and Ward [16].
Maximum strength assessment
Maximum strength was assessed using the deadlift exercise
test, using an Olympic bar and 1.5 kg to 20 kg weight plates. The test involved
warming up with 1 set of 10 repetitions with 40 to 60% of the estimated
one-repetition maximum (1RM), and, after 1 min, another set of 5 repetitions
with 60 to 80% of the estimated 1RM, and after 3 min, five attempts at a
maximum voluntary action were performed until a 1RM for each subject was
identified [17]. Rests (approximately 4-5 minutes) were taken between attempts to
maintain maximum performance.
Aerobic capacity assessment
Aerobic capacity was indirectly assessed using the Yo-Yo
Intermittent Recovery Level I (Yo-Yo) test. Running in a demarcated space
consists of running a distance of 20 m twice (‘round trip’ = 40 m), separated
by regular recovery periods of 10 seconds. The time to run the 40 m was
progressively reduced, representing higher speeds at each stage. The subjects
were instructed to complete as many stages as possible, reaching the end of the
course at each sound signal. The test was interrupted when the volunteer was
unable to complete the stage (being more than 3 m before the 20 m line on two
consecutive sound signals) or reported an inability to complete the run. The
test was performed in one attempt. Based on the distance and speed achieved
during the test, aerobic capacity was determined, expressed by maximum oxygen
consumption (VO2max) in ml/kg/min (formula for predicting VO2max = distance x
0.0084 + 36.4). The choice of Yo-yo Intermittent Recovery Level I was based on
the study by Bangsbo, Iaia, and Krustrup [18].
Intervention: physical training
The type of exercise adopted as an intervention in the
present study was a CrossFit® WOD known as ‘Cindy’ training [19,20].
The session began
with a warm-up consisting of 5 min of low-intensity running and 5 min of joint
mobility and dynamic stretching exercises. This WOD consisted of performing as
many rounds as possible (AMRAP) of three exercises: 5 repetitions of pull-ups,
10 repetitions of push-ups, and 15 repetitions of air squats for 20 min.
To characterize exercise intensity, during the ‘Cindy’
training, subjects were monitored using a heart rate (HR) monitor (Polar Team
Pro, Kempele, Finland). HR
data were stored and later extracted from the Polar Team 2 Pro program. Additionally, the subjective perception of exertion
(RPE), which has been strongly recommended for use in metabolic HIFT sessions
[21,22,23], was obtained using the CR10 Borg scale [24]. Participants answered the
following question: ‘How hard did you think the exercise was?’. RPE measurement
was performed 30 min after the ‘Cindy’ workout.
Standardization of breakfast on the day of data collection – visit 5
One hour before the training session, volunteers consumed
powdered food supplements mixed with water as a standardized breakfast
(approximately 320–350 calories). The breakfast consisted of protein, fat, and
carbohydrates in the proportions of 20-35-45 (percentage of protein, fat, and
carbohydrate). These percentages culminated in the ingestion of approximately
40 g of carbohydrates, 17.5 g of protein, and 13 g of fat. To avoid gastric
discomfort on the day of the intervention, the same breakfast was offered in
the familiarization session with the training protocol. The standardized
breakfast was determined by a nutritionist specialized in sports nutrition.
Biochemical analyses
The biomarkers adopted in the present study were
thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD),
glutathione peroxidase (GPx), and ferric-reducing antioxidant power (FRAP).
Determination of lipid peroxidation by the TBARS method
The determination of lipid peroxidation in plasma was
performed by quantification of TBARS, according to the method described by
Ohkawa et al. [25], with minor modifications. A standard curve of 1,
1', 3, 3'-Tetraethoxypropane - TEP (0.5 - 8.0 nmol) was prepared, and the
results were expressed in nmol of TEP/mL of plasma.
Antioxidant capacity via FRAP (Ferric-Reducing Antioxidant Power) method
Plasma was used to determine total antioxidant capacity
according to the method described by Benzie and Strain [26] in 96-well plates
using the FRAP reagent. Absorbance was read at 595 nm, and the results were
expressed in mM eq. of Trolox/mL of plasma.
Determination of superoxide dismutase activity
SOD activity was assessed according to the methodology of
McCord and Fridovich [27], which verifies the production of superoxide anion
produced by the xanthine/xanthine oxidase system. The superoxide anion produced
reduced cytochrome C, and this reduction was measured by the increase in
optical density at 550 nm at 25ºC. The results were expressed in U/g of
hemoglobin. One unit (U) is considered to be the activity of the enzyme that
promotes 50% inhibition of the xanthine reaction at 25ºC at pH 7.8.
Determination of glutathione peroxidase activity
GPx activity was determined using the standardized
methodology developed by Sies et al. [28]. This method is based on the
measurement of the optical density decay at 340 nm caused by the oxidation of
NADPH at 30 °C during the reduction of oxidized glutathione (GSSG) catalyzed by
the enzyme glutathione reductase. The results were expressed in U/g of
hemoglobin. One unit (U) of the enzyme was defined as the activity of the
enzyme that oxidizes 1 µmol of NADPH per minute at 30°C at pH 7.0.
Statistical analysis
Normality and homogeneity of variances were verified by
the Shapiro-Wilk and Levene tests, respectively. Data are presented as mean and
standard deviation. The paired t-test (times) was used to compare the mean
values of descriptive variables of all subjects. Two-way repeated measures
analysis of variance (ANOVA) (group interaction [EXP x NOV and MEN x WOM] ×
time) was used to compare blood analyses, followed by the Bonferroni post hoc
test to identify differences. All analyses were performed with SPSS-22.0
software (IBM, SPSS Inc., Chicago, IL, USA). Significance was set at p <
0.05.
Ethical approvals
Ethical approval was obtained from the Research Ethics
Committee (Research Ethics Committee of the Federal University of Sergipe,
process no. 3,087,955/2018). Participation was voluntary, and all participants
signed the Free and Informed Consent Form before participating in the study.
Results
The training session adopted in the present study was
performed at high intensity, as shown in Table II.
Table II - Characterization of the intervention -
Cardiovascular responses, performance, and perceived exertion of participants
undergoing the ‘Cindy’ protocol (M ± SD)

M = mean; SD = standard deviation; HRx = average heart
rate; bpm = beats per minute; %HRmax = percentage of maximum heart rate; RPE =
subjective perception of effort
According to Figure 2, there was a significant reduction
(p < 0.05) in SOD after 30 min when compared with the values observed in the
pre-exam. GPx and FRAP showed a significant increase (p < 0.05) after 30
minutes. There were no changes in TBARS (Table III).
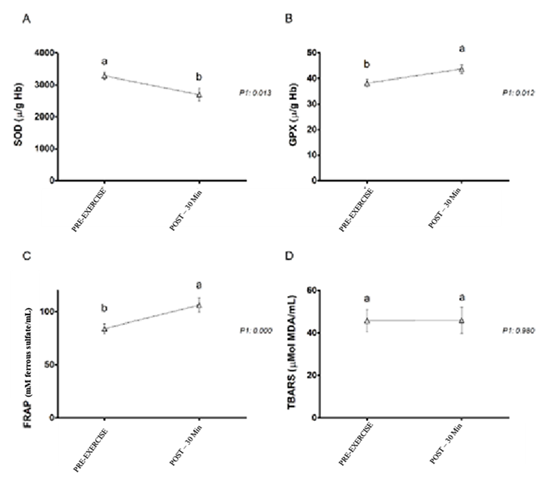
Letters compare means across time points; means followed
by different letters are statistically different (p ≤ 0.05), while means
followed by the same letter or not followed by any letter do not differ (p ≤
0.05). P1: effect of time ; SOD = superoxide dismutase; GPx = glutathione
peroxidase; FRAP = ferric-reducing antioxidant power; TBARS = thiobarbituric
acid reactive substances
Figure 2 - Variation of SOD (A), GPx (B), and FRAP (C) in 19
participants and TBARS (D) in 11 participants. Comparisons between pre-exercise
and post-exercise (POST-30min) moments
Table III - Evaluation of biomarkers at different times for
both sexes (M ± SD)
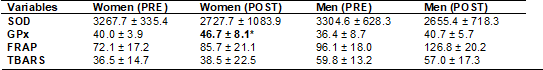
M = mean; SD = standard deviation; SOD = Superoxide
dismutase; GPx = Glutathione peroxidase; FRAP = Ferric-reducing ability of
plasma; TBARS = thiobarbituric acid reactive substances
The comparison of results between genders showed that GPx
increased significantly in women (time effect), with no differences between
genders.
Table IV - Assessment of biomarkers at different times for
different levels of experience (M ± SD)
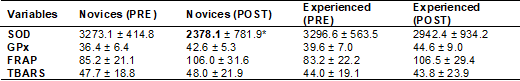
M = mean; SD = standard deviation; SOD = Superoxide
dismutase; GPx = Glutathione peroxidase; FRAP = Ferric-reducing ability of
plasma; TBARS = thiobarbituric acid reactive substances
The comparison of results between genders showed that SOD
was significantly reduced in novices (time effect) (Table IV).
Discussion
The main findings of the present study showed that a
high-intensity ‘Cindy’ training session promoted an increase in antioxidant
capacity (FRAP), a significant reduction in SOD, and a notable increase in GPx,
without changes in TBARS.
The increase in FRAP levels was also observed in the
study by Kliszczewicz et al. [6]; this phenomenon is expected after a
high-intensity activity, whether predominantly aerobic or anaerobic [29], which
corroborates the exercise protocol adopted in the present study, since our
intervention was shown to be high-intensity, through monitoring of HR and RPE.
The increase in FRAP after intense exercise has been explained by the joint and
efficient work of the various antioxidants available in the body of its
practitioners, which increase their efforts to combat the more significant
production of free radicals caused by exercise [30].
When evaluating antioxidant activity, we observed an
increase in GPx after 30 minutes of training. This fact can be explained by the
concept that antioxidant enzymes respond adaptively, increasing their
activities to combat free radicals and the damage caused by them after physical
exercise, especially high-intensity exercise [31]. Santos et al. [32]
also reported an increase in GPx after high-intensity exercise (RAST TEST) by
trained athletes; the authors justified this increase as an attempt by the
antioxidant system to combat the oxidative stress generated by the RAST TEST.
During physical exercise, including activities in which
the metabolism is predominantly anaerobic, or in isometric or explosive
exercises, such as in CrossFit®, ischemia and reperfusion occur. In tissue
reperfusion, O2, together with hypoxanthine, promotes the synthesis of
superoxide anion (O2-) and hydrogen peroxide (H2O2), species with a high
reactive content [33]. Thus, in situations where there is an increase in
pro-oxidant compounds, GPx acts together with SOD in an attempt to convert
superoxide anion and hydrogen peroxide into water: through dismutation, SOD,
being zinc-dependent, catalyzes the synthesis of hydrogen peroxide from
superoxide anion; however, as hydrogen peroxide is reactive and can promote
oxidative damage, GPx, a selenium-dependent enzyme, catalyzes the reduction of
hydrogen peroxide into water (Figure 3) [34]. Thus, in the present study, GPx
was likely elevated to counter a potential increase in hydrogen
peroxide-induced by Cindy.
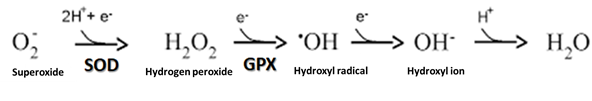
Figure 3 - Conversion of superoxide radical into water,
through the action of SOD and GPx enzymes. Adapted from: Barreiros, David, and
David (2006) [35]
It is worth noting that the increase in hydrogen peroxide
may be the result of the action of SOD on the superoxide radical (Chart 1).
Therefore, GPx would ‘continue’ the work of SOD. According to Groussard et
al. [36], the accumulation of hydrogen peroxide can reduce SOD activity,
corroborating our findings, which demonstrated a drop in SOD 30 minutes after
exercise.
Considering this hypothesis, it is possible to assume
that, in the present study, at some point during and immediately after Cindy,
SOD activity may have increased in an attempt to combat a possible increase in
superoxide radicals, as has been observed in recent studies [33,37,38]; the
action of SOD would culminate in greater formation of H2O2, and subsequently,
the accumulation of H2O2 would have inhibited the action of SOD, characterizing
the drop in SOD observed 30 min after the end of Cindy. However, to test this
hypothesis, it would have been necessary to assess SOD activity at more time
points than those tested in our study, which can be considered a limitation.
Another limitation is that the nutritional status of the
participants with zinc and selenium was not assessed since possible
deficiencies of these nutrients could interfere with the activity of the SOD
and GPx enzymes, respectively [39].
There is also the hypothesis that the reduction in SOD
could indicate that our intervention (Cindy) was not intense enough to cause
significant production of reactive oxygen species, not requiring an increase in
SOD activity to eliminate excess superoxide [40]. However, in this case, GPx
activity and FRAP would not have been elevated either, which weakens this
hypothesis. Likewise, the %HR and RPE data also rule out this hypothesis since
both methods indicated high intensity.
Finally, in our study there was no change in TBARS
concentrations, these compounds being considered markers of lipid peroxidation.
Evaluating this result together with the other findings of the present study,
it is possible to suggest the hypothesis that exercise (Cindy) caused greater
formation of oxidative compounds, which were efficiently ‘combated’ by the
participants’ antioxidant system, which in this study was evaluated through SOD
and GPx, but which may have been strengthened by numerous other mechanisms not
assessed in the present study. All these antioxidant efforts would have
culminated in an increase in the antioxidant capacity (FRAP), and this stronger
defense would have been sufficient to prevent lipid peroxidation, thus
justifying the maintenance of TBARS.
The impact of exercise sessions on the TBARS
concentration of its practitioners has been the subject of numerous studies,
and the results are divergent, with some studies corroborating our findings
[36,41,42,43,44] and others in which TBARS were elevated [45,46]. This divergence is
understandable since it is believed that, in organisms in which antioxidant
defenses are efficient, for several reasons, including nutritional status and
sufficient to combat the reactive species generated by intense physical exercise,
there will be greater protection of membrane lipids, culminating in less
damage, marked by TBARS. On the other hand, in situations where antioxidant
defenses are inefficient and situations where the formation of reactive species
generated by intense physical exercise is very high, it will not be possible to
‘guarantee’ the protection of membrane lipids, culminating in greater damage,
marked by elevated TBARS [47].
The low reliability and accuracy of the TBARS assessment
method have also been considered a possible cause of the contradictory results
in the literature [42, 48]. It is also important to highlight that in the assay
to assess lipid peroxidation (TBARS), there was a partial loss of blood
samples, and, for this reason, we had only 11 participants, a small population,
which may increase the risk of bias.
Additionally, there is still the possibility that changes
in TBARS after exercise occur later, beyond the 30 minutes adopted in the
present study. However, to test this hypothesis, it would be necessary to have
evaluated TBARS more times than those tested in our investigation, which can be
considered a limitation.
As discussed previously, GPx reduces oxidative stress to
prevent oxidative damage. Thus, according to Fortes et al. [49], women
and men have significant physiological differences. For example, men's muscle
fibers are larger than women's, which is why men tend to excel in exercises
that require speed and strength. In addition, they have a lower fatigue
threshold than women. Furthermore, in a certain study, women were shown to be
more susceptible to muscle injuries caused by oxidative stress. Therefore, it
can be thought that GPx increased only in women since they need a higher level
of effort to reproduce the same exercise as men, consequently increasing ROS,
oxidative stress, and GPx.
Regarding the decrease in SOD in the novice group, we can
argue that the physical capacity of the experienced ones is more developed
[50]. Therefore, novices need greater effort to perform the same exercises,
thus increasing the level of oxidative stress and, consequently, superoxide
anion molecules. However, as previously discussed, it may be that at the time
of analysis, the biomarker in question had its levels reduced by the strong
presence of hydrogen peroxide, which has been shown to decrease SOD concentrations.
Additionally, according to Powers et al. [9],
exercise intensity depends on the functionality of the individual's
cardiovascular system and fatigue, so the limitation of the cardiovascular
system, with fatigue, alters the intensity and duration of the exercise. Thus,
concerning novices, SOD decreased when compared to experienced ones. It can be
assumed that the duration and intensity of the exercise were lower in this
group, and after 30 minutes, there were no longer as many superoxides to be
combatted. However, more blood samples and SOD analysis at different times
would be necessary to confirm the hypothesis.
Finally, the oxidative stress caused by physical
exercise, depending on its level, can be beneficial or harmful to health and
performance [9]. Thus, analyzing our results, because the protocol applied was
of high intensity, we saw that there was an increase in oxidative stress caused
by the intensity of the physical exercise, but we did not find evidence of
oxidative damage 30 minutes after its end. Thus, our findings suggest that
performing Cindy would not increase the risk of undesirable outcomes, such as
muscle injuries, loss of strength, impaired hypertrophy, or development of
chronic non-communicable diseases.
As far as we have investigated, this is the first study
to analyze the acute effects of a HIFT session on these biomarkers of oxidative
stress. For this reason, our results may be useful to verify how the redox
state of an individual behaves in a CrossFit® workout to assess whether or not
there is oxidative stress and, in the future, to encourage more in-depth
discussions on the hypothesis that this modality is related to an increased
risk of injuries. Such future discussions are fundamental; after all, according
to Moran et al. [51], there has been an exponential increase in the
number of HIFT practitioners worldwide.
Conclusion
A Cindy session promoted an increase in GPx and FRAP, a
reduction in SOD, and maintenance of TBARS in CrossFit® practitioners, 30
minutes after its completion. GPx changes were observed in women, while SOD
changes were observed among novices. FRAP was altered in all groups.
Conflicts of interest
There are no conflicts of interest.
Sources of funding
No funding.
Authors' contributions
Conception and design of the research: Bastos BMB, Gomes JH, Silva AMO, Mendes RR; Data collection: Gomes
JH, Mendes RR; Data analysis and interpretation: Bastos BMB, Gomes JH,
Silva AMO, Mendes RR; Statistical analysis: Gomes JH; Manuscript
writing: Bastos BMB, Mendes RR; Critical revision of the manuscript for
important intellectual content: Bastos BMB, Gomes JH, Mendes RR
References
- Gomes JH, Mendes RR, Franca
CS, Da Silva-Grigoletto ME, Pereira da Silva DR,
Antoniolli AR, et al. Acute leucocyte, muscle damage, and stress marker
responses to high-intensity functional training. PLoS One. 2020 Dec
3;15(12):e0243276. doi: 10.1371/journal.pone.0243276 [Crossref]
- Ben-Zeev T, Okun E. High-Intensity Functional Training: Molecular Mechanisms and Benefits. Neuromolecular Med. 2021 Sep 1;23(3):335–8. doi: 10.1007/s12017-020-08638-8 [Crossref]
- Timón R, Olcina G, Camacho-Cardeñosa M, Camacho-Cardenosa A, Martinez-Guardado I, Marcos-Serrano M. 48-hour recovery of biochemical parameters and physical performance after two modalities of CrossFit workouts. Biol Sport. 2019;36(3):283–9. doi: 10.5114/biolsport.2019.85458 [Crossref]
- Barranco-Ruiz Y, Villa-González E, Martínez-Amat A, Da Silva-Grigoletto ME. Prevalence of injuries in exercise programs based on Crossfit®, Cross training and high-intensity functional training methodologies: a systematic review. J Hum Kinet. 2020;73:251–65. doi: 10.2478/hukin-2020-0006 [Crossref]
- Bergeron MF, Nindl BC, Deuster PA, Baumgartner N, Kane SF, Kraemer WJ, et al. Consortium for health and military performance and American College of Sports Medicine Consensus Paper on extreme conditioning programs in military personnel. Curr Sports Med Rep. 2011;10(6):383–9. doi: 10.1249/JSR.0b013e318237bf8a [Crossref]
- Kliszczewicz B, John QC, Daniel BL, Gretchen OD, Michael ER, Kyle TJ. Acute exercise and oxidative stress: CrossFitTM vs. Treadmill Bout. J Hum Kinet. 2015;47(1):81–90. doi: 10.1515/hukin-2015-0064 [Crossref]
- Meyer J, Morrison J, Zuniga J. The benefits and risks of crossfit: a systematic review. Workplace Health Saf. 2017;65(12):612–8. doi: 10.1177/2165079916685568 [Crossref]
- Minghelli B, Vicente P. Musculoskeletal injuries in Portuguese CrossFit practitioners. J Sports Med Phys Fitness. 2019;59(7). doi: 10.23736/S0022-4707.19.09367-8 [Crossref]
- Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: Friend or foe? J Sport Health Sci. 2020;9(5):415–25. doi: 10.1016/j.jshs.2020.04.001 [Crossref]
- Juturu V, Sahin K, Pala R, Tuzcu M, Ozdemir O, Orhan C, et al. Curcumin prevents muscle damage by regulating NF-kB and Nrf2 pathways and improves performance: an in vivo model. J Inflamm Res. 2016;9:147–54. doi: 10.2147/JIR.S110873 [Crossref]
- Cheng AJ, Jude B, Lanner JT. Intramuscular mechanisms of overtraining. Redox Biol. 2020;35:101480. doi: 10.1016/j.redox.2020.101480 [Crossref]
- Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med. 2013;19(1):101–6. doi: 10.1038/nm.3019 [Crossref]
- Butcher S, Judd T, Benko C, Horvey K, Pshyk A. Relative intensity of two
types of crossfit exercise: acute circuit and high-intensity interval exercise.
Journal of Fitness Research [Internet]. 2015;4(2):3-15. [cited 2024 August 12].
Available from: https://www.researchgate.net/publication/281240359_Relative_Intensity_of_Two_Types_of_CrossFit_Exercise_Acute_Circuit_and_High-Intensity_Interval_Exercise
- Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. 2018;15(1). doi: 10.1186/s12970-018-0242-y [Crossref]
- Jackson AS, Pollock ML. Generalized
equations for predicting
body density of men. Br J Nutr. 1978;40(3):497–504. doi: 10.1079/BJN19780152 [Crossref]
- Jackson AS, Pollock ML, Ward
A. Generalized equations
for predicting body density
of women. Med Sci
Sports Exerc. 1980;12(3):175–81. Available from:
https://pubmed.ncbi.nlm.nih.gov/7402053/
- Dias RMR, Avelar A, Menêses AL, Salvador EP, Silva DRP da, Cyrino ES. Segurança, reprodutibilidade, fatores intervenientes e aplicabilidade de testes de 1-RM. Motriz: Revista de Educação Física. 2013 Mar;19(1):231–42. doi: 10.1590/S1980-65742013000100024 [Crossref]
- Bangsbo J, Iaia FM, Krustrup P. The Yo-Yo Intermittent Recovery Test. Sports Medicine. 2008;38(1):37–51. doi: 10.2165/00007256-200838010-00004 [Crossref]
- Glassman G. Metabolic Conditioning. The CrossFit Journal Article
[Internet]. 2003;(3):1–4. [cited 2024 April 15]. Available from:
https://brokenscience.org/wp-content/uploads/2024/06/Jun03_metab_cond.pdf
- Kliszczewicz B, Snarr R, Esco M. Metabolic and cardiovascular response to the crossfit workout “Cindy”: a pilot study. J Sport Human Perf. 2014;2(2). doi: 10.12922/jshp.v2i2.38 [Crossref]
- Tibana R, Sousa N, Prestes J, Voltarelli F. Lactate, heart rate and rating of perceived exertion responses to shorter and longer duration CrossFit® training sessions. J Funct Morphol Kinesiol. 2018;3(4):60. doi: 10.3390/jfmk3040060 [Crossref]
- Falk Neto JH, Tibana RA, de Sousa NMF, Prestes J, Voltarelli FA, Kennedy MD. Session rating of perceived exertion is a superior method to monitor internal training loads of functional fitness training sessions performed at different intensities when compared to training impulse. Front Physiol. 2020;11. doi: 10.3389/fphys.2020.00919 [Crossref]
- Tibana RA, Sousa NMF, Prestes J, Nascimento DC, Ernesto C, Falk JH, et al. Is perceived exertion a useful indicator of the metabolic and cardiovascular responses to a metabolic conditioning session of functional fitness? Sports. 2019;7(7):161. doi: 10.3390/sports7070161 [Crossref]
- Foster C, Florhaug JA, Franklin J, Gottschall L, Hrovatin LA, Parker S, et
al. A new approach to monitoring exercise training. J Strength Cond Res.
2001;15(1):109–15. Available from: https://pubmed.ncbi.nlm.nih.gov/11708692/
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. doi: 10.1016/0003-2697(79)90738-3 [Crossref]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239(1). doi: 10.1006/abio.1996.0292 [Crossref]
- McCord JM, Fridovich
I. Superoxide dismutase. An enzymic function
for erythrocuprein (hemocuprein). J Biol Chem. 1969;244(22). Available from:
https://pubmed.ncbi.nlm.nih.gov/5389100/
- Sies H, Koch OR, Martino E, Boveris A. Increased biliary glutathione disulfide release in chronically ethanol-treated rats. FEBS Lett. 1979;103(2). doi: 10.1016/0014-5793(79)81346-0 [Crossref]
- Quindry J, Miller L, McGinnis G, Kliszczewiscz B, Slivka D, Dumke C, et al. Environmental temperature and exercise-induced blood oxidative stress. Int J Sport Nutr Exerc Metab. 2013;23(2). doi: 10.1123/ijsnem.23.2.128 [Crossref]
- Panza VP. Efeito do consumo de chá verde no
estresse oxidativo em praticantes de exercício resistido [Dissertação]. Florianópolis:
UFSC; 2007. [cited 2024 July 20]. Available from:
https://bdtd.ibict.br/vufind/Record/UFSC_59147e25852b7bdbc40a5a3c3cd39f28
- Araújo MB, Prada FJA, Mello
MAR. Estresse oxidativo no exercício, modelos animais e intensidade do esforço
Conceito de Estresse Oxidativo. Motriz, Rio Claro. 2006;12. Available
from:
https://www.periodicos.rc.biblioteca.unesp.br/index.php/motriz/article/view/423
- Santos PMF, Souza LMV, Santos MB, Araújo JES, Santos JL, Dória IB, et al. The acute effect of rast test on oxidative stress and muscle damage markers in young athletes. J Phys Educ. 2018;29(1). doi: 10.4025/jphyseduc.v29i1.2980 [Crossref]
- Petry E, Alvarenga M, Cruzat V, Tirapegui J. Exercício físico e estresse oxidativo: mecanismos e efeitos. Revista Brasileira de Ciência e Movimento. 2010;18(4):90-99. doi: 10.18511/rbcm.v18i4.1363 [Crossref]
- Barbosa KBF, Costa NMB, Alfenas RCG, Paula SO, Minim VPR, Bressan J. Estresse oxidativo: conceito, implicações e fatores modulatórios. Rev Nutr. 2010;23(4):629–43. doi: 10.1590/S1415-52732010000400013 [Crossref]
- Barreiros ALBS, David JM, David JP. Estresse oxidativo: relação entre geração de espécies reativas e defesa do organismo. Quim Nova. 2006;29(1):113–23. doi: 10.1590/S0100-40422006000100021 [Crossref]
- Groussard C, Rannou-Bekono F, Machefer G, Chevanne M, Vincent S, Sergent O, et al. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur J Appl Physiol. 2003;89(1):14–20. doi: 10.1007/s00421-002-0767-1 [Crossref]
- Gonçalves ÁC, Rodrigues LR,
Terra MP, Sasaki JE, Portari GV. Exercício aeróbio exaustivo aumenta o estresse
oxidativo em corredores fundistas treinados. RBPFEX - Revista Brasileira de
Prescrição e Fisiologia do Exercício [Internet]. 2019;13(83):493–500.
[cited 2024 Aug 12]. Available from:
http://www.rbpfex.com.br/index.php/rbpfex/article/view/1704
- Vazatta R, Tangerino LCS, Araújo GG, Melo MP, Cavaglieri CR, Verlengia R. Exercício físico e mecanismo antioxidante de defesa. Saúde em Revista. 2009;11(28–29):7–15. doi: 10.15600/2238-1244/sr.v11n28-29p7-15 [Crossref]
- Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biology. 2020. doi: 10.1016/j.redox.2020.101508 [Crossref]
- Boeno FP, Ramis TR, Farinha JB, Lemos LS de, Medeiros N da S, Ribeiro JL. Efeito agudo do exercício de força com restrição do fluxo sanguíneo sobre parâmetros antioxidantes em indivíduos jovens saudáveis. J Vasc Bras. 2018;17(2). doi: 10.1590/1677-5449.011017 [Crossref]
- Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc. 2000;1576–81. doi: 10.1097/00005768-200009000-00008 [Crossref]
- Morillas-Ruiz J, Zafrilla P, Almar M, Cuevas MJ, López FJ, Abellán P, et al. The effects of an antioxidant-supplemented beverage on exercise-induced oxidative stress: results from a placebo-controlled double-blind study in cyclists. Eur J Appl Physiol. 2005;95(5–6):543–9. doi: 10.1007/s00421-005-0017-4 [Crossref]
- Morillas-Ruiz JM, Villegas García JA, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clinical Nutrition. 2006;25(3):444–53. doi: 10.1016/j.clnu.2005.11.007 [Crossref]
- Spanidis Y, Stagos D, Orfanou M, Goutzourelas N, Bar-or D, Spandidos D, et al. Variations in oxidative stress levels in 3 days follow-up in ultramarathon mountain race athletes. J Strength Cond Res. 2017;31(3):582–94. doi: 10.1519/JSC.0000000000001584 [Crossref]
- Lima D, Voltarelli
F, Kietzer K. Verificação de um biomarcador de
estresse oxidativo em atletas de natação em período específico de treinamento
físico. Revista Brasileira de Prescrição e Fisiologia do Exercício [Internet]. 2015;9:97–104. [cited 2024 Aug 15]. Available from:
https://www.rbpfex.com.br/index.php/rbpfex/article/view/746
- Varamenti E, Tod D, Pullinger SA. Redox homeostasis and inflammation responses to training in adolescent athletes: a systematic review and meta-analysis. Sports Med Open. 2020;6(1):34. doi: 10.1186/s40798-020-00262-x [Crossref]
- Thirupathi A, Pinho RA, Ugbolue UC, He Y, Meng Y, Gu Y. Effect of running exercise on oxidative stress biomarkers: a systematic review. Front Physiol. 2021;11. doi: 10.3389/fphys.2020.610112 [Crossref]
- Rodriguez MC, Rosenfeld J, Tarnopolsky MA. Plasma malondialdehyde increases transiently after ischemic forearm exercise. Med Sci Sports Exerc. 2003 Nov;35(11):1859–65. doi: 10.1249/01.MSS.0000093609.75937.70 [Crossref]
- Fortes M, Marson R, Martinez
E. Comparação de desempenho físico entre homens e mulheres: revisão de
literatura. Revista Mineira de Educação Física [Internet]. 2015;23:54–69.
Available from:
https://periodicos.ufv.br/revminef/article/view/9964
- Hilário G, Abdalla PP,
Carvalho A dos S, Júnior JRG. Condicionamento físico de participantes de
crossfit®. RBPFEX - Revista Brasileira de Prescrição e Fisiologia do Exercício
[Internet]. 2022;16:173–81. [cited 2024 August 15]. Available from:
http://www.rbpfex.com.br/index.php/rbpfex/article/view/2541
- Moran S, Booker H, Staines J, Williams S. Rates and risk factors of injury in CrossFitTM: a prospective cohort study. J Sports Med Phys Fitness. 2017 Jul;57(9). doi: 10.23736/S0022-4707.16.06827-4 [Crossref]
