Rev Bras Fisiol Exerc. 2024;23(2):e235604
doi: 10.33233/rbfex.v23i2.5604OPINIÃO
Methodological challenges in randomized clinical trials in physical
education: the design of non-inferiority
Desafios metodológicos
em ensaios clínicos randomizados na educação física: o design de não
inferioridade
Antônio Marcos Andrade Costa1,2,3,
Ewerton de Souza Bezerra2, Nathalia Bernardes3
1Universidade Estadual do Amazonas (UEA),
Manaus, AM, Brazil
2Universidade Federal do Amazonas (UFAM),
Manaus, AM, Brazil
3Universidade São Judas Tadeu, São
Paulo, SP, Brazil
Received: June 10,
2024; Accepted: July 18, 2024
Correspondence: Antonio
Marcos Andrade Costa, antoniomarcoshand@gmail.com
How to cite
Antônio Marcos Andrade Costa
AMA, Ewerton de Souza Bezerra ES, Nathalia Bernardes N. Methodological
challenges in randomized clinical trials in physical education: the design of non-inferiority. Rev Bras Fisiol exerc.
2024;23(2):e235604. doi: 10.33233/rbfex.v23i2.5604
Abstract
The Physical
Education professional, like any
health professional, needs to make decisions during the exercise
of his professional activity. These decisions must be prudent, aiming for the greatest
benefit for your client. In this context, randomized clinical trials (RCTs) are
considered the gold standard to guide decision making. In this context,
randomized clinical trials (RCTs) are considered the gold standard to guide
decisions. However, mistaken judgments can occur when interpreting the results
of clinical superiority studies, because they assume that two interventions are
identical due to the absence of statistical difference, however, the lack of
statistical significance does not support the conclusion of equality; that is,
the absence of evidence is not evidence of absence. In this scenario, an
elegant alternative is equivalence and non-inferiority studies, which should be
used whenever a new intervention has a substantial practical advantage compared
to the old, already established one. According to the methodological strategy,
a tolerance margin for non-inferiority is established using the limits of the
confidence interval. In this way, once non-inferiority has been demonstrated,
we become more convinced that the intervention will bring the expected benefit
to our client. Therefore, our proposal was to draw attention to this
methodological technique that can be of great use in our area and that needs to
be further explored.
Keywords: physical education; randomized clinical trial;
non-inferiority.
Resumo
O profissional de Educação
Física, como qualquer profissional de saúde, necessita tomar decisões durante o
exercício da sua atividade profissional. Essas decisões devem ser prudentes
visando o maior benefício para o seu cliente. Neste contexto, os ensaios
clínicos randomizados (ECR) são considerados o padrão ouro para orientar a
decisão. No entanto, julgamentos equivocados podem acontecer na interpretação
dos resultados de estudos clínicos de superioridade, isto porque assumem que
duas intervenções são idênticas devido a ausência de
diferença estatística, todavia, a falta de significância estatística não apoia
a conclusão da igualdade; isto é, a ausência de evidência não é evidência de
ausência. Neste cenário, uma alternativa elegante são os estudos de
equivalência e não inferioridade, que devem ser utilizados sempre que uma nova
intervenção tenha uma vantagem prática substancial em comparação com a antiga
já estabelecida. De acordo com a estratégia metodológica, é estabelecida uma
margem de tolerância para não inferioridade utilizando os limites do intervalo
de confiança. Dessa forma, uma vez demonstrada a não inferioridade, ficamos
mais convencidos que a intervenção trará benefício esperado para nosso cliente.
Portanto, nossa proposta foi chamar a atenção para essa técnica metodológica
que pode ser de grande utilidade em nossa área e que necessita ser mais
explorada.
Palavras-chave: educação física; ensaios clínicos
randomizados; não-inferioridade.
Introduction
The Physical Education professional, like any other
health professional, needs to make decisions during the exercise of their
clinical activity. These decisions must be prudent and most likely to benefit
your patient client. To achieve this, the mental process of judgment that
precedes your actions must be based on logical analysis that follows a mental
trigger, taking into account your professional expertise, the patient and the
evidence regarding the conduct you intend to take. Well-planned and executed clinical
trials are the best methodological designs for testing the cause and effect
relationship between a set of independent and dependent variables in
experimental models [1].
When it comes to interventions involving human subjects,
randomized controlled trials (RCTs) are considered by proponents of
evidence-based healthcare to be the gold standard design to guide
decision-making [2]. Classically, sample groups are defined through random
allocation, with one being an experimental group (representing the intervention
being tested) and another group being considered the control – which can
sometimes be no treatment, a placebo or, more frequently, a recognized efficacy
treatment. The results undergo appropriate statistical analysis in order to
validate the conclusions and identify the best interventions. This
methodological model is called effectiveness superiority study or comparative
effectiveness, and its analysis for decision making involves testing
hypotheses; the null hypothesis we call H0, and the alternative hypothesis
called H1. In this type of experiment, the randomization process, when carried
out satisfactorily, makes the groups homogeneous and therefore comparable, eliminating
confounding factors, which leads the investigator to reject H0 in the presence
of a p value < 0.05 , and conclude that the difference observed between the
groups comes from the intervention applied.
Frequentist statistics teaches us that, by not rejecting
the null hypothesis, we may be facing what we call a type II error – failing to
show a relevant difference due to the lack of appropriate sizing at the time of
designing the study. This normally occurs under conditions of low statistical
power, either due to an insufficient number of participants or due to biases in
the design and/or conduct of the study [3]. In superiority study designs, the
null hypothesis (H0) states that the intervention tested is not superior to the
control group, while the alternative hypothesis (H1) states that the
intervention is superior to the control group.
However, we observe misinterpretations when it is not
possible to reject the null hypothesis, as it is not uncommon for researchers
to conclude that in the absence of statistical difference between
interventions, they are equal. Many authors report their results in a way that
leads readers to conclude that the interventions “are equivalent”, one way to
identify this practice is when faced with a finding in which it was not
possible to identify a difference, the authors begin to base their narratives
solely on biological plausibility, inducing the reader to extract a positive
result in the absence of significance [4]. However, the lack of statistical
significance does not support the conclusion of equality between interventions;
that is, 'the absence of evidence is not evidence of absence [5]. Inference
errors like these have been appearing more frequently and can contribute to the
formation of a flawed and unreliable ecosystem.
It is in this scenario that an elegant alternative emerges to test a
promising idea that presents a clear practical advantage (low cost, lower risk,
low application complexity, among others) which are non-inferiority projects.
In this construct, the null hypothesis (H0) states that the new intervention
tested is not inferior to the control (old), therefore similar, or that through
a plausible a priori argument, it is accepted until the new intervention is
less effective (it is established a non-inferiority limit), so that (H0) and
the conclusion of non-inferiority can be accepted. In an attempt to materialize
a concrete example, below is figure 1, taken from the study “Non-inferiority
clinical trials: concepts and issues” [6].
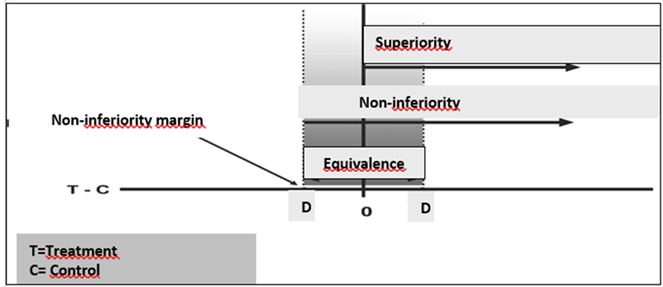
T = treatment; C = control. T is superior to C if the
confidence interval of the difference lies entirely to the right of zero,
non-inferior if entirely to the right of -D, and equivalent if contained within
the equivalence zone between -D and +D
Figure 1 - Theoretical basis for concepts applied during RCT
An ideal moment to resort to non-inferiority studies is
when a new intervention emerges or when testing something new is necessary.
However, the candidate must offer an explicit advantage over the intervention
already established in the literature, justifying the acceptance of
non-inferiority. An example of an intervention that, in our understanding,
would justify testing non-inferiority is high-intensity interval training
(HIIT) in the outcome of improving VO2max. Once demonstrated in a
non-inferiority design that HIIT can achieve the non-inferiority threshold, we
can conclude that the intervention is indeed time-efficient.
In table I, it was reprinted from the study by Pinto [6], in 2010, in which
the author presents an analysis algorithm for three types of hypothetical
studies, in which T represents the measure of effectiveness of the new
intervention, and C the measure of effectiveness of the control group.
Rejecting the null hypothesis means, for superiority studies, that the new
intervention called T is superior to the control group C; non-inferiority
studies, when the difference between C and T is smaller than a margin delta (D)
non-inferiority margin and, for equivalence studies, that the difference
between C and T is neither smaller nor larger than a margin D. Fundamentally,
the term equivalent means non-inferior and non-superior, and testing for
equivalence refers to the analysis for the symmetric region defined by [+D,-D].
Table I - Formulation of hypotheses for superiority,
non-inferiority, and equivalence studies

Effectiveness measures are presented in the table above
as; T-new intervention, C- control, and D as the margin of
non-inferiority/equivalence
Moreover, several factors must be carefully considered
when planning, analyzing, and interpreting non-inferiority studies to ensure
the study's internal validity: a) choice of the non-inferiority margin; b) the
number of participants required for the study; c) control of the study's
sensitivity; d) definition of the analysis population. Some other factors
should be considered as well, but they are beyond the scope of our discussion
at the moment.
Conclusion
In the field of research in Physical Education, as well
as in other segments of the health sector, we need to pay attention to make the
best decisions, and knowing how to interpret the results of scientific findings
is an elementary skill, essential to becoming better professionals. Our
intention was to alert the academic community and science consumers regarding
the conclusion of similarity drawn from the results of clinical trials of
superiority of efficacy without statistical difference. As we frequently
observe, many professionals justify the application of an intervention based on
the inferences drawn from this mental model, which makes the debate relevant
and necessary.
We highlight non-inferiority studies as an alternative to
address this issue, as we believe it is a more elegant methodological
technique, as it establishes specific parameters to test similarity or a limit
to accept non-inferiority. For those who wish to delve deeper into the topic,
we recommend two reference materials that were valuable in preparing this
reflection: one developed by the CONSORT [7] group and the other provided by
the Canadian Cancer Partnership at McMaster University [8]. Bringing this topic
into a broader discussion and encouraging the development of specific
guidelines and guidelines on the subject appears to be an emerging need.
Funding
This research did not receive any specific grant from
funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of
interest statement
The authors have no conflict of interests
to declare.
Referências
- Portney LG. Experimental Designs. In: Foundations of clinical research:
Application to evidence-based practice. 4.ed.
Philadelphia, USA: FA Davis Company; 2020.
- Zabor EC, Kaizer
AM, Hobbs BP. Randomized Controlled Trials. Chest. 2020;158(1):S79-87. doi: 10.1016/j.chest.2020.03.013 [Crossref]
- Baldissera R. Uma iniciação
aos testes estatísticos para dados biológicos: inclui scripts dos testes em R.
Chapecó: UFFS; 2022.
- Boutron I, Dutton S, Ravaud P, Altman DG. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA. 2010;303(20):2058–64. doi: 10.1001/jama.2010.651 [Crossref]
- Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ 1995;311:485
- Pinto VF. Ensaios clínicos
de não inferioridade: conceitos e questões. Jornal Vascular
Brasileiro. 2010;145-51.
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group.
Reporting of noninferiority and equivalence randomized trials: an extension of
the CONSORT statement. JAMA. 2006;295:1152-60.
- Canadian Partnership Against Cancer. Capacity Enhancement Program: non-inferiority and equivalence trials checklist [Internet]. McMaster University; 2009. [citado 2024 set 13]. http://fhs.mcmaster.ca/cep/documents/CEPNon-inferiorityandEquivalenceTrialChecklist.pdf
