Rev
Bras Fisiol Exerc. 2025;24:e245621
ORIGINAL ARTICLE
Nonpharmacological strategies against hypertension:
Effect of resistance training and acclimation on cardioprotection
Estratégias
não farmacológicas contra a hipertensão arterial: efeito do treinamento
resistido e aclimatação na cardioproteção
Jéssica
da Silva Santos, Ronaldo André Castelo dos Santos de Almeida, Letícia de Sousa
Amorim, Emerson Lopes Olivares, Anderson Luiz Bezerra da Silveira
Universidade Federal Rural do
Rio de Janeiro, Seropédica, Rio de Janeiro, Brazil
Received: January 30, 2025; Accepted: March 13, 2025.
Correspondence: Emerson
Lopes Olivares, olivares.el@gmail.com
How to cite
Santos JS, Almeida RACS, Amorim LS, Olivares EL, Silveira
ALB. Nonpharmacological
strategies against hypertension: Effect of resistance training and acclimation
on cardioprotection. Rev Bras Fisiol Exerc. 2025;24:e245621. doi: 10.33233/rbfex.v24i1.5621
Introduction: Hypertension
(HT) is the main risk factor for myocardial infarction. Together, these events
are the main causes of death worldwide. The conventional treatment is
pharmacological. Non-pharmacological strategies, such as resistance training
(RT) and heat acclimation (HA), may affect reducing cardiovascular risk. Objective:
The present study aimed to evaluate the effects of RT and HA on ventricular
function, systolic blood pressure (SBP), and cardioprotection
of spontaneously hypertensive rats (SHR). Methods: The experimental
procedures were authorized under registration number 14/2022 (CEUA/ICBS/UFRRJ).
SHR were divided into a control group (CTR, n = 7), a group trained 3x/week/10
weeks (TG, n = 8), and a group acclimated in a heated bath for 11 consecutive days
(HWI, n = 9). SBP was assessed by tail plethysmography. Left ventricular
function (LVF) was evaluated by the isolated heart method. Cardioprotection
assessment was based on LVF in the 60 min after global ischemia (IQ = 30 min)
and on the analysis of the infarct area. Results: After the trials, only
CTR showed higher SBP (p < 0.01). Left ventricular developed pressure (LVDP)
was better during reperfusion in the HWI groups compared to CTR (p < 0.05)
and TG (p < 0.05). The infarct area after IQ was smaller only in HWI (p <
0.05). Conclusion: TG and HWI demonstrated an effect on maintaining and
reducing SBP in the experimental groups, but only HWI was effective in
promoting cardioprotection.
Keywords: hypertension; resistance training; heat acclimation; cardioprotection; health.
Resumo
Introdução: A hipertensão arterial (HA) é o
principal fator de risco para o infarto do miocárdio. Juntos, estes eventos são
as principais causas de morte no mundo. O tratamento convencional é o
farmacológico. Estratégias não farmacológicas, como o treinamento resistido
(TR) e a aclimatação ao calor (ACC), podem ter efeito na redução do risco
cardiovascular. Objetivo: O presente estudo teve como objetivo
avaliar os efeitos do TR e da ACC sobre a função ventricular, pressão arterial
sistólica e cardioproteção de ratos espontaneamente
hipertensos (SHR). Métodos: Os procedimentos experimentais foram
autorizados sob registro n° 14/2022
(CEUA/ICBS/UFRRJ). SHR foram divididos em grupo controle (CTR, n = 7), grupo
treinado 3x/semana/10 semanas (TR, n = 8) e grupo aclimatado em banho aquecido
por 11 dias consecutivos (HWI, n = 9). A pressão arterial sistólica (PAS) foi
avaliada por pletismografia de cauda. A função ventricular esquerda (FVE) foi
avaliada pelo método de coração isolado. A avaliação de cardioproteção
baseou-se na FVE nos 60min após isquemia global (IQ = 30 min) e na análise da
área de infarto. Resultados: Após os ensaios, apenas CTR apresentou maior PAS (p < 0,01). A pressão
desenvolvida pelo ventrículo esquerdo (PDVE) foi melhor durante a reperfusão
nos grupos HWI comparados a CTR (p < 0,05) e TR (p < 0,05). A área de
infarto após IQ foi menor apenas em HWI (p < 0,05). Conclusão: TR e
HWI demonstraram efeito na manutenção e redução da PAS nos grupos
experimentais, mas apenas HWI foi efetivo na promoção da cardioproteção.
Palavras-chave: hipertensão; treinamento
resistido; aclimatação ao calor; cardioproteção;
saúde
Introduction
Cardiovascular diseases lead the ranking
of causes of death in the world
The conventional treatment for hypertension is
pharmacological. This works through different mechanisms of action (diuretics,
angiotensin-converting enzyme inhibitors, angiotensin II AT1 receptor blockers,
among others). However, the use of these drugs generates negative effects on the
health and life quality
Seeking to minimize these side effects, new therapies
have been proposed to control and reduce SBP exclusively or as adjuvant
therapies
Individually, these strategies have not yet
demonstrated their effect on the heart after ischemia and reperfusion injury
(IRI), as well as on the extent of the infarct area and cardioprotection.
The impossibility of performing invasive procedures in humans led us to use an
experimental model to carry out these evaluations. Therefore, in this study, we
used spontaneously hypertensive rats (SHR), animals that present a hypertensive
phenotype analogous to that of human hypertension. This phenotype is due to
their genetic predisposition
The hypothesis that the trained group (TG) and the
group acclimated to heat in a heated bath (HWI) would be able to improve cardioprotection and better recovery of ventricular
function after IRI was tested. The aim of the study was to verify the effect of
TG and HWI on SBP and infarct area after 30 min of ischemia in SHR hearts.
Methods
Animals
SHR,
males, were randomly divided into control (CTR, n = 9), heat-acclimated (HWI, n
= 9), and resistance trained (TG, n = 9) groups. Experimental procedures were
approved by the Ethics Committee for Animal Use of the Institute of Biomedical
and Health Sciences of UFRRJ (CEUA 14/2022/ICBS/UFRRJ).
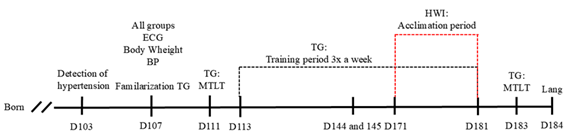
ECG = electrocardiogram; MTLT = maximum transported
load test; TG = trained group; HWI = heat acclimated group; Lang = Langendorff
isolated heart protocol
Figure 1 - Experimental design. D, day. RT, resistance training.
BP, blood pressure
Familiarization
with the training apparatus
The rats in the TG group were
familiarized with the resistance training apparatus to minimize failures in the
execution of the training. The familiarization routine is described in Table I.
Table I - Adaptation protocol for resistance training apparatus

Resistance training (RT) was performed on a ladder
with a height of 110 cm and an 80º inclination
The test was performed on up to two consecutive
days. On the first day, the animals were forced to climb the ladder carrying an
initial load of 50% of their body mass (BM). The load was attached to the end
of a cable fixed to the chest vest. If the climb was successful, the load was
increased by 10% of the BM on the subsequent climbing attempt for a second
attempt. If the climb was successful again, the load was increased by another
5% of the BM. If the climb was still successful on this last attempt of the
day, the test was restarted on the following day, adding 5% to the last load
carried. This was repeated up to a maximum of the third attempt on the second
day. All animals reached their maximum load by the second day. Failure was
determined when the animal was unable to climb the ladder after three
consecutive stimuli on the tail (with a tweezer), with a rest interval of 120 s
between each climb.
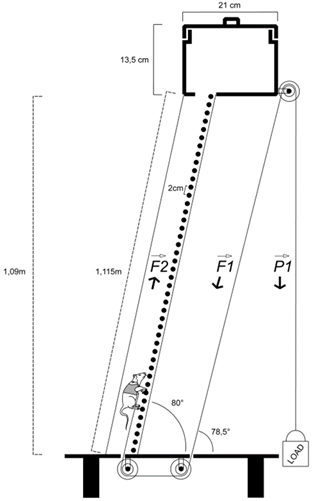
The
load pulled by the animal corresponds to the initial load (LOAD) at the
opposite end of the pulley as they are fixed pulleys
Figure 2 - Training apparatus using a vest
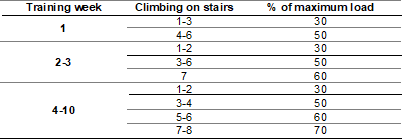
Training protocol
The
training load was calculated based on the individual maximum load (load of the
last complete climb) for each rat. The exercise load was adjusted in the fifth
week according to the new maximum test load. RT was performed 3 days/week, for
10 weeks with a load of 30-70% of the maximum load. The rats performed 6 to 8
climbs, depending on the training week. A 1-minute interval between climbs was
used
Table II - Resistance training protocol on
the stairs with pulley and vest
Acclimation protocol
Rats in
the HWI group were subjected to a hot bath for eleven days, starting on the
first day with a 20-minute stay and adding 5 minutes each day, until reaching
60 minutes on the ninth day. From the ninth to the eleventh day, the session
was 60 minutes long. The hot bath was performed in a pool for rats with a water
temperature of 40 ºC.
SBP
measurement
A noninvasive tail cuff measurement
system was used to acquire systolic blood pressure (SBP) (Digital Tail
Plethysmograph with Dual-Channel Heater, Bonther, Ribeirão Preto, SP, Brazil), previously validated by Feng et al. [20]. This system detects SBP
based on volume changes in the tail
ECGs were performed before and
after the experimental protocol 3 days before the start of the protocol. The
animals remained for 10-15 min with the electrodes so that they could adapt to
the equipment and, in this way, any noise during the ECG at the beginning and
end of the experiment would be reduced. An analog-digital interface (Power Lab
400, ADInstruments, United States of America - USA)
was used for data collection and the data were stored on a computer for later
analysis. Data analysis was performed using Lab Chart 8 Pro software (ADInstruments, United States of America). The complementary
modules ECG Analysis 2.4 (ADInstruments, USA) and
Heart Rate Variability 2.0 (HRV 2.0, ADInstruments,
USA) were used for ECG analysis.
Isolated
heart protocol
The hearts were removed and
connected to the Langendorff apparatus by inserting a cannula with a continuous
flow of 10 ml.min-1 into the aorta, characterizing retrograde flow
of this technique. The artificial perfusion solution used was the modified
Krebs-Henseleit (KHS) containing 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25
mM NaHCO3, 10 mM C6H12O6, 1.8 mM CaCl2, saturated with a carbogenic mixture (95% O2 + 5% CO2).
The solution was adjusted to pH 7.4 and kept warm at 37 ºC, being pumped with a
continuous flow through the circuit through the perfusion pump. A latex balloon
connected to a pressure transducer was inserted through the left atrium. The
balloon was filled with distilled water and a pressure of 10 mmHg was adjusted.
Through an amplifier (ML110, ADInstruments, United
States of America), it was possible to record the intraventricular pressure
developed by the left ventricle through an analog-digital interface (PowerLab 400, ADInstruments,
United States of America) and stored with the aid of software for analysis of
biological signals (Lab Chart 8 Pro, ADInstruments,
United States of America).
Morphometry
Measurement
of the infarct area
The apices were discarded, and
slices were prepared in sections of approximately 1 mm in thickness from the
apex to the base for mounting on slides. To improve the contrast between viable
and necrotic tissues, the slices were incubated in 1% triphenyltetrazolium
chloride in phosphate buffer (pH 7.4) for 5 min at 37°C and then incubated in
10% formaldehyde solution for 24 h. The sections were placed between two glass
slides, and their images were digitally acquired using a scanner (Lide 300 USB,
Cannon, Brazil). The infarct size was determined using ImageJ software (public
domain, version 1.54k).
Statistics
Data
were presented as mean ± standard error of the mean (SEM). The Shapiro-Wilk
test was used to verify the normality of measurements. One-way analysis of
variance (ANOVA) was used to analyze continuous response variables and
categorical explanatory variables. Two-way ANOVA with Sidak's post-test was
used for temporal analysis of ex-vivo data. When normality in the distribution
was not verified, the Wilcoxon paired rank test was used. GraphPad Prism 10.1.1
software (GraphPad Software, USA) was used for the analyses. Statistical
differences were considered significant when the significance value was less
than 5% (p < 0.05).
Results
Pre-experimental
morphometry (in vivo)
The
analysis of body mass before the tests showed lower mass in the TG: 252.7 ± 4.8
g (95% CI = 240.2 to 265.1)) compared to CTR (CTR: 299.8 ± 9.3 g (95% CI =
277.7 to 321.8 g)), p < 0.001) and HWI (HWI: 298.4 ± 3.0 g (95% CI = 291.5
to 305.4 g, p < 0.001)). At the end of the experiment, with access to the
tibia length, TG equaled CTR and decreased the difference in HWI (F (2, 24) =
3.863; p = 0.0373), demonstrating a significant gain in BM resulting from the
training performed.
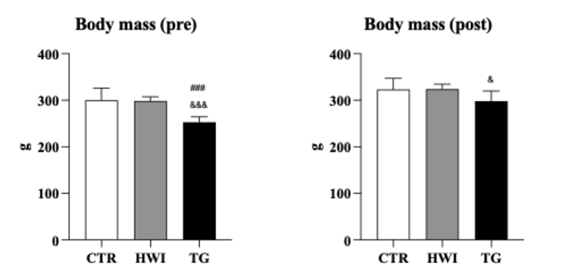
Data are expressed as mean ± SEM. & TG vs HWI (p =
0.0373); &&&TG vs HWI (p = 0.0002); ###TR vs CTR (p = 0.0002). CTR
= Control; HWI = Acclimatizing; TG = Trained group
Figure 3 - Pre- and post-experimental morphometry between groups
Systolic blood pressure (SBP)
At the
end of the experimental protocol, SBP in HWI (200.2 ± 5.4 mmHg, 95% CI = 187.7
to 212.7 mmHg) and TG (206.4 ± 8.9 mmHg, 95% CI = 185.9 to 226.9 mmHg) remained
constant, while CTR (235.4 ± 8.8 mmHg, 95% CI = 213.9 to 257) showed an
increase in SBP, as expected for this particular strain (F (2, 24) = 5.253; p =
0.0128).
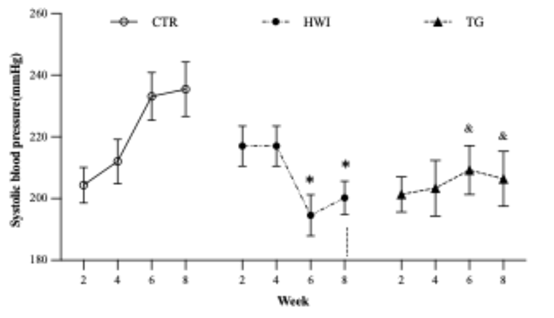
HWI reduced SBP from week 8 compared to CTR and
from week 10 compared to TG. *HWI vs CTR (p = 0.0065); &TG vs CTR (p =
0.0364). CTR = Control; HWI = Acclimating; TG = Trained Group
Figure 4 - Systolic blood pressure
ECG
The ECG was used to assess heart rate (HR) before and
after the experimental intervention. It was observed that both CTR (pre: 378.0 ± 18.4
bpm, post: 434.2 ± 11.8 bpm, p = 0.0196) and HWI (pre: 370.8 ± 15.9
bpm, post: 432.1 ± 12.5 bpm, p = 0.0149) had an increase in HR comparing
their pre vs post moments. TG showed a reduction in HR after the experimental
protocol (pre: 393.3 ± 11.4 bpm, post: 354.7 ± 12.4
bpm, p = 0.0281).
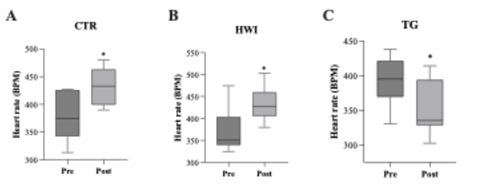
Data
are expressed as mean ± SEM. *(CTR: p = 0.0196; HWI: p = 0.0149; TG: p =
0.0281). CTR = Control; HWI = Acclimating; TG = Trained Group; Pre =
pre-experimental; Post = post-experimental
Figure 5 - Intragroup heart rate
Hemodynamics
Left ventricular performance was not different between
groups at baseline (p = 0.4601). During the ischemic period (20-50 min), HWI
and TG had an attenuated reduction in contractility in the first 5 min (HWI vs
CTR: p = 0.0451; TG vs CTR: p = 0.0324), which suggests cellular energy
savings. During reperfusion, only HWI had better recovery of ventricular
function (LVDP) compared to CTR and TG (F (2, 24) = 4.631; p = 0.0216).
Table III – Ventricular performance
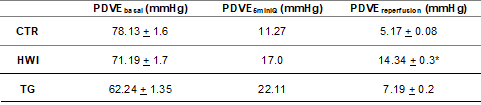
Left ventricular
pressure developed at baseline, LVDPbasal;
Left ventricular pressure developed at 5 minutes of GA, LVDP5minIQ;
Left ventricular pressure developed during reperfusion, LVDPreperfusion;
Data are expressed as mean ± SEM. CTR = Control group; HWI = Heat acclimated
group; TG = Trained group. *p = 0.0216
Ex-vivo morphometry
Table IV – Ex-vivo cardiac
morphometry

*CTR vs HWI; #CTR
vs TG; P < 0.0001
Analysis of the infarct area
No
differences were observed in the contractility index between the groups (p =
0.2392). The infarct area was smaller in HWI compared to CTR (p = 0.0129) and TG
(p = 0.0446).
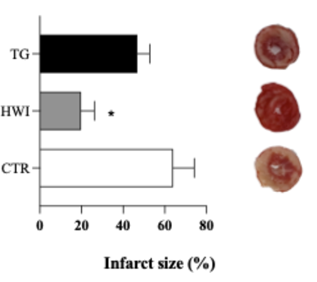
A = Infarct area; B =
Representative images. Data are expressed as mean ± SEM. *p = 0.0110. CTR =
Control; HWI = Acclimating; TG = Trained Group
Figure 6 - HWI reduced the infarct area,
represented as a percentage of the total area
Discussion
Among the groups, TG had the lowest body mass before
the interventions. After the trials, the body mass of TG did not differ from
CTR and HWI (p > 0.05). This increase demonstrated the effect of training
and suggests the hypertrophic effect, even though it was not the main objective
of this study.
SHR rats have the characteristic of gradually
increasing their SBP throughout their lives
Post-exercise hypotension in humans has already been
explained by the withdrawal of sympathetic tone and central vasodilation
through NO
Heat acclimation through sauna bathing has already
been described as an acute hypotensive therapy
The effects of the training protocol and heat
acclimation on hemodynamics were evident in the first 5 min of the IQ period,
when TG and HWI presented higher LVDP values. This fact demonstrates that the
myocytes of both groups sustained some degree of contractility for longer,
without nutrition. There was no significant difference in the contractility
index between the groups, therefore the ischemic contracture was not attenuated
by acclimation or resistance training. In the reperfusion period, HWI
demonstrated better recovery.
The increased cardiac mass in TG
reflects the effect of training. This expected adaptation did not result in
better cardiac performance or even better recovery after IQ. Thus, no evidence
of cardioprotection was observed in this group.
Analysis of the infarct area demonstrated a smaller area of
infarcted tissue after 60 min of reperfusion in HWI hearts. This
observation, together with the better recovery of left ventricular function in
the HWI group, demonstrates the cardioprotection
conferred by the acclimatization protocol to which the animals were subjected.
The mechanism related to this cardioprotection
involves the action of the response to thermal stress mediated by heat shock
proteins
Limitations
Although SBP is measured using the inflatable cuff
method, which is non-invasive and less stressful for the animal, this technique
does not yet provide a reliable measurement for diastolic blood pressure, in
addition to depending on a period of pre-experimental familiarization of the
animals with the apparatus, in order to avoid or at least reduce the stress of
contraction during the measurement. We chose this technique to meet the demand
of the 3Rs principle and to reduce the number of animal lives in the experiment
performed.
We used only male animals, but the use of females
would be of great value to expand the conclusions and clarify any questions
dependent on sex.
Another factor that we observed throughout the
experiments in our laboratory and that was more evident in this study was the
use of 30 minutes of IQ. We believe that this period can be reduced so that we
can better assess the recovery of the hearts during the reperfusion period and
prevent viable hearts from being lost due to excessive IQ time.
Conclusion
According to the data obtained in this study, we
observed that the resistance training protocol decreased heart rate and
stabilized SBP in SHR rats, while heat acclimatization conferred cardioprotection in SHR rats, which had not yet been
demonstrated in the literature. Taken together, these data demonstrate the
potential effect of training and acclimatization, which, if performed in a
planned manner, can promote health by reducing cardiovascular risk. Further
studies are needed to verify the exact mechanisms of cardioprotection
observed.
Acknowledgments
The authors would like to thank
the Department of Physiological Sciences of UFRRJ for providing the
infrastructure for the study through the Laboratory of Cardiovascular
Physiology and Pharmacology
Conflicts of interest
The authors declare no
conflicts of interest.
Sources of funding
Santos JS is a CNPq scientific initiation fellow (135961/2024-6). Almeida
RACS is a CNPq doctoral fellow (140562/2023-0).
Amorim LS is a CNPq scientific initiation fellow
(135506/2024-7).
Authors' contributions
Conception
and design of the research: Santos JS, Almeida
RACS; Data collection: Santos JS, Almeida RACS, Amorim LS;
Acquisition of data: Santos JS, Amorim LS; Analysis and interpretation of the data: Santos JS, Almeida RACS; Statistical
analysis: Almeida RACS;
Obtaining financing: Olivares EL; Writing of the manuscript: Santos JS, Almeida RACS; Critical
revision of the manuscript for important intellectual content: Silveira ALB, Olivares EL.
References
- Roth GA, Abate D, Abate KH, Abay
SM, Abbafati C, Abbasi N,
et al. Global, regional, and
national age-sex-specific mortality for 282 causes of death in 195 countries
and territories, 1980–2017: a systematic analysis for the Global Burden of
Disease Study 2017. The Lancet [Internet]. 2018;392(10159):1736–88. Available
from: https://linkinghub.elsevier.com/retrieve/pii/S0140673618322037
- Carvalho MV,
Siqueira LB, Sousa AL, Jardim PC. The influence of hypertension on
quality of life. Arq Bras Cardiol.
2013;100(2):164-74. doi: 10.5935/abc.20130030 [Crossref]
- Kjeldsen SE et al. Hypertension and cardiovascular risk: General
aspects. Pharmacol Res. 2018;129:95-99. doi: 10.1016/j.phrs.2017.11.003 [Crossref]
- Nosalski R, McGinnigle E, Siedlinski M, Guzik TJ. Novel immune mechanisms in hypertension and cardiovascular risk. Curr Cardiovasc Risk Rep. 2017;11(4):12. doi: 10.1007/s12170-017-0537-6 [Crossref]
- Barroso WKS, Rodrigues CIS, Bortolotto LA, Mota-Gomes MA, Brandão AA, Feitosa ADM, et al. Brazilian guidelines of hypertension - 2020. Arq Bras Cardiol. 2021;116(3):516–658. doi: 10.36660/abc.20201238 [Crossref]
- Heidari B, Avenatti E, Nasir K. Pharmacotherapy for essential
hypertension: a brief review. Methodist Debakey
Cardiovasc J. 2022;18(5)5-16. doi: 10.14797/mdcvj.1175 [Crossref]
- Mensah GA, Bakris G. Treatment and control of high blood pressure in adults. Cardiol Clin. 2010;28(4):609-22. doi: 10.1016/j.ccl.2010.08.002 [Crossref]
- Gheorghe A, Griffiths U, Murphy A, Legido-Quigley H, Lamptey P, Perel P. The economic burden of cardiovascular disease and hypertension in low- and middle-income countries: A systematic review. BMC Public Health. 2018;18(1):975. doi: 10.1186/s12889-018-5806-x [Crossref]
- Dixon DL, Johnston K, Patterson J, Marra CA, Tsuyuki RT. Cost-Effectiveness of pharmacist prescribing for managing hypertension in the United States. JAMA Netw Open. 2023;6(11):E2341408. doi: 10.1001/jamanetworkopen.2023.41408 [Crossref]
- Nogueira IC, Santos ZMSA, Gardano D, Mont´alverne B, Barbosa A. Effects of exercise on hypertension control in older adults: systematic review. Rev Bras Geriatr Gerontol. 2012;15:3. doi: 10.1590/S1809-98232012000300019 [Crossref]
- Gomes MFP, Borges ME, Rossi VA, Moura EOC, Medeiros A. The effect of physical resistance training on baroreflex sensitivity of hypertensive rats. Arq Bras Cardiol. 2017;108(6):539–45. doi: 10.5935/abc.20170065 [Crossref]
- Baffour-Awuah B, Pearson MJ, Dieberg G, Smart NA. Isometric resistance training to manage hypertension: systematic review and meta-analysis. Curr Hypertens Rep. 2023;25:35–49. doi: 10.1007/s11906-023-01232-w [Crossref]
- Henkin JS, Pinto RS, Machado CLF, Wilhelm EN. Chronic effect of resistance training on blood pressure in older adults with prehypertension and hypertension: A systematic review and meta-analysis. Exp Geront. 2023;177:112193. doi: 10.1016/j.exger.2023.112193 [Crossref]
- Fernandes AA, Faria TO, Júnior RFR, Costa GP, Marchezini B, Silveira EA, et al. A single resistance exercise session improves myocardial contractility in spontaneously hypertensive rats. Braz J Med Biol Res. 2015;48(9):813–21. doi: 10.1590/1414-431X20154355 [Crossref]
- Laukkanen JA, Jae SY, Kunutsor SK. The interplay between systolic blood pressure, sauna bathing, and cardiovascular mortality in middle-aged and older Finnish men- a cohort study. J Nutr Health Aging. 2023;348–53. doi: 10.1007/s12603-023-1895-1 [Crossref]
- Doris PA. Genomic and “Polyomic” Studies of Cardiovascular and Inflammatory Diseases Genetics of hypertension: an assessment of progress in the spontaneously hypertensive rat. Physiol Genomics. 2017;49:601–17. doi: 10.1152/physiolgenomics.00065.2017 [Crossref]
- Fazan V, Kalil A,
Alcântara A, Genari A, Tavares M, Rodrigues A, et al.
usp. Medicina (B Aires). 2006;39:39–50. [cited 2024 Nov 12]. Available from:
https://repositorio.usp.br/item/001534448
- Duncan ND, Williams DA, Lynch GS. Adaptations in rat skeletal muscle following long-term resistance exercise training. Eur J Appl Physiol Occup Physiol. 1998;77(4):372-8. doi: 10.1007/s004210050347 [Crossref]
- Neves RVP, Souza MK, Passos CS, Bacurau RFP, Simoes HG, Prestes J, et al. Resistance training in spontaneously hypertensive rats with severe hypertension. Arq Bras Cardiol. 2016;106(3):201–9. doi: 10.5935/abc.20160019 [Crossref]
- Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008 21(12):1288–91. doi: 10.1038/ajh.2008.301 [Crossref]
- Wilde E, Aubdool AA, Thakore P, Baldissera L, Alawi KM, Keeble J, et al. Tail-cuff technique and its influence on central blood pressure in the mouse. J Am Heart Assoc. 2017;6(6). doi: 10.1038/ajh.2008.301 [Crossref]
- Okamoto K, Aoki K.
Development of a Strain of Spontaneously Hypertensive Rats. Jpn
Circ J [Internet]. 1963;27(3):282–93. [cited 2024 Nov 12]. Available from:
http://www.jstage.jst.go.jp/article/circj1960/27/3/27_3_282/_article
- Melo RM, Martinho E, Michelini LC. Training-induced, pressure-lowering effect in SHR: wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension. 2003;42(4):851–7. doi: 10.1161/01.HYP.0000086201.27420.33 [Crossref]
- Miura S. Evidence for exercise therapies including isometric handgrip training for hypertensive patients. Hyperten Res. 2024;48:846-8. doi: 10.1038/s41440-024-02033-7 [Crossref]
- Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(Suppl 1):S193–S201. doi: 10.1038/sj.bjp.0706458 [Crossref]
- Teodoro BG, Natali JA, Fernandes SAT, Peluzio MCG. A influência da intensidade do exercício físico aeróbio no processo aterosclerótico. Rev Bras Med Esporte. 2010;16(5). doi: 10.1590/S1517-86922010000500013 [Crossref]
- Banks NF, Rogers EM, Stanhewicz AE, Whitaker KM, Jenkins NDM. Resistance exercise lowers blood pressure and improves vascular endothelial function in individuals with elevated blood pressure or stage-1 hypertension. Am J Physiol Heart Circ Physiol. 2024;326(1):H256–69. doi: 10.1152/ajpheart.00386.2023 [Crossref]
- Philip L, Jose A,
Basit H, Lappin SL. Physiology, Starling Relationships [Internet]. [cited 2024
Nov 14]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459390/?report=printable
- Barauna VG, Magalhaes FC, Krieger JE, De Oliveira EM. AT1 receptor participates in the cardiac hypertrophy induced by resistance training in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(2). doi: 10.1152/ajpregu.00933.2007 [Crossref]
- Basford JR. The
Law of Laplace and its relevance to contemporary medicine and rehabilitation.
Arch Phys Med Rehabil [Internet]. 2002;83(8):1165–70.
[cited 2024 Nov 23]. Available from:
https://linkinghub.elsevier.com/retrieve/pii/S0003999302000436
- Romero SA, Minson CT, Halliwill JR. The cardiovascular system after exercise. Exercise J Appl Physiol. 2017;122:925–32. doi: 10.1152/japplphysiol.00802.2016 [Crossref]
- Halliwill JR, Minson CT, Joyner MJ, Joyner MCJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol. 2000;89(5):1860-6. doi: 10.1152/jappl.2000.89.5.1830 [Crossref]
- Arakawa K, Miura SI, Koga M, Kinoshita A, Urata H, Kiyonaga A. Activation of renal by physical dopamine system exercise. Hypertens Res. 1995:18(Suppl1): S73-7. doi: 10.1291/hypres.18.supplementi_s73 [Crossref]
- Hannuksela ML, Ellahham S. Benefits and risks of sauna bathing. Am J Med. 2001;110(2):118–26. doi: 10.1016/s0002-9343(00)00671-9 [Crossref]
- Gayda M, Bosquet L, Paillard F, Garzon M, Sosner P, Juneau M, et al. Effects of sauna alone versus postexercise sauna baths on short-term heart rate variability in patients with untreated hypertension. J Cardiopulm Rehabil Prev. 2012;32(3):147–54. doi: 10.1097/HCR.0b013e318251ffeb [Crossref]
- Kunutsor SK, Jae SY, Kurl S, Laukkanen JA. Sauna bathing and mortality risk: unraveling the interaction with systolic blood pressure in a cohort of Finnish men. Scand Cardiol J. 2024;58(1). doi: 10.1080/14017431.2024.2302159 [Crossref]
- Rodrigues P, Orssatto LBR, Gagnon D, Dahhak A, Hecksteden A, Stewart IB, et al. Passive heat therapy: a promising preventive measure for people at risk of adverse health outcomes during heat extremes. J Appl Physiol. 2024:136;677–94. doi: 10.1152/japplphysiol.00701.2023 [Crossref]
- Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol. 2016;594(18):5329–42. doi: 10.1113/JP272453 [Crossref]
- Horowitz M, Assadi H. Heat acclimation-mediated cross-tolerance in cardioprotection: do HSP70 and HIF-1alpha play a role? Ann N Y Acad Sci. 2010;1188:199–206. doi: 10.1111/j.1749-6632.2009.05101.x [Crossref]
